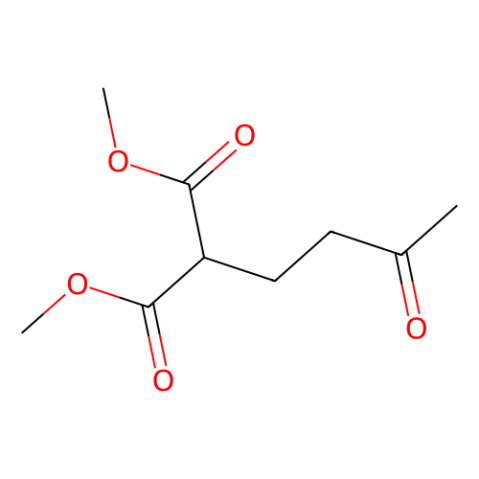
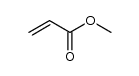
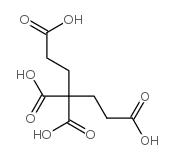
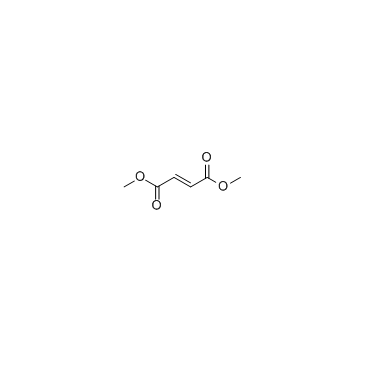
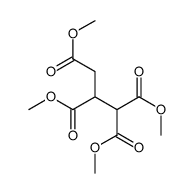
|
~91% |
|
~56% |
|
~94% |
|
~94% |
|
~96% |
|
~83% |

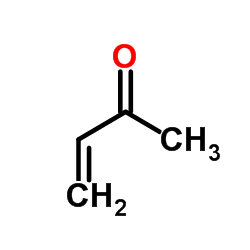
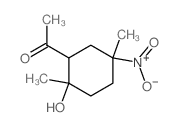


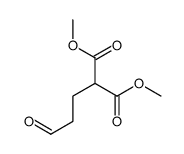
![dimethyl 2,2-bis[2-(benzenesulfonyl)ethyl]propanedioate Structure](https://image.chemsrc.com/caspic/319/181186-14-1.png)