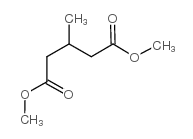
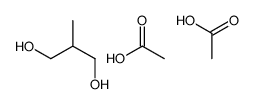
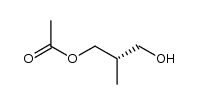
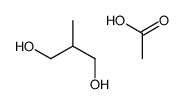
|
~83% |
|
~0% |
|
~59% |
|
~59% |