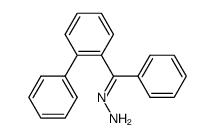
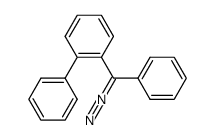
|
~62% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~31% |
|
~81% |
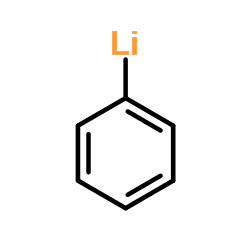
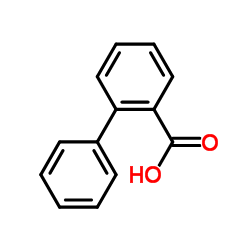
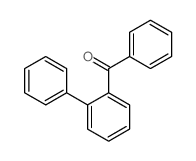
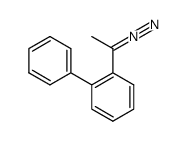
![[1,1'-Biphenyl]-2-methanol,a-methyl Structure](https://image.chemsrc.com/caspic/202/16927-84-7.png)
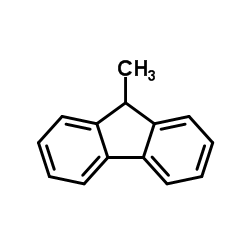
![1-([1,1'-Biphenyl]-2-yl)ethanone Structure](https://image.chemsrc.com/caspic/270/2142-66-7.png)
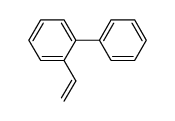

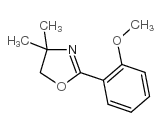
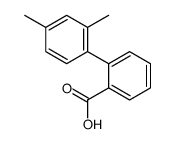
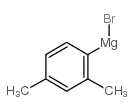
![2-(2',4'-dimethyl-[1,1'-biphenyl]-2-yl)-4,4-dimethyl-4,5-dihydrooxazole Structure](https://image.chemsrc.com/caspic/399/183313-47-5.png)

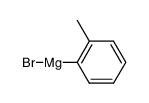
![2'-Methyl-[1,1'-Biphenyl]-2-Carboxylic Acid Structure](https://image.chemsrc.com/caspic/399/7111-77-5.png)
![4,4-dimethyl-2-[2-(2-methylphenyl)phenyl]-5H-1,3-oxazole Structure](https://image.chemsrc.com/caspic/007/156641-63-3.png)