Influence of nucleotide identity on ribose 2'-hydroxyl reactivity in RNA.
Kevin A Wilkinson, Suzy M Vasa, Katherine E Deigan, Stefanie A Mortimer, Morgan C Giddings, Kevin M Weeks
Index: RNA 15(7) , 1314-21, (2009)
Full Text: HTML
Abstract
Hydroxyl-selective electrophiles, including N-methylisatoic anhydride (NMIA) and 1-methyl-7-nitroisatoic anhydride (1M7), are broadly useful for RNA structure analysis because they react preferentially with the ribose 2'-OH group at conformationally unconstrained or flexible nucleotides. Each nucleotide in an RNA has the potential to form an adduct with these reagents to yield a comprehensive, nucleotide-resolution, view of RNA structure. However, it is possible that factors other than local structure modulate reactivity. To evaluate the influence of base identity on the intrinsic reactivity of each nucleotide, we analyze NMIA and 1M7 reactivity using four distinct RNAs, under both native and denaturing conditions. We show that guanosine and adenosine residues have identical intrinsic 2'-hydroxyl reactivities at pH 8.0 and are 1.4 and 1.7 times more reactive than uridine and cytidine, respectively. These subtle, but statistically significant, differences do not impact the ability of selective 2'-hydroxyl acylation analyzed by primer extension-based (SHAPE) methods to establish an RNA secondary structure or monitor RNA folding in solution because base-specific influences are much smaller than the reactivity differences between paired and unpaired nucleotides.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
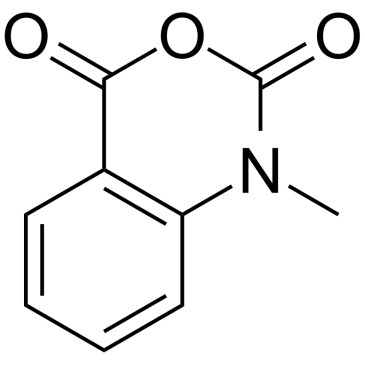 |
N-Methylisatoic Anhydride
CAS:10328-92-4 |
C9H7NO3 |
|
Structure and function of echinoderm telomerase RNA.
2016-02-01 [RNA 22 , 204-15, (2016)] |
|
Simultaneous determination of myo-inositol and scyllo-inosit...
2007-04-01 [Electrophoresis 28(8) , 1221-8, (2007)] |
|
Role of lysine binding residues in the global folding of the...
2015-01-01 [RNA Biol. 12 , 1372-82, (2016)] |
|
RNA structure analysis at single nucleotide resolution by se...
2005-03-30 [J. Am. Chem. Soc. 127(12) , 4223-31, (2005)] |
|
RNA SHAPE chemistry reveals nonhierarchical interactions dom...
2005-04-06 [J. Am. Chem. Soc. 127(13) , 4659-67, (2005)] |