Journal of Organic Chemistry
2001-09-07
Regio- and diastereoselective synthesis of 2-alkylidenetetrahydrofurans by domino SN/SN' and SN/SN reactions of 1,3-dicarbonyl dianions.
P Langer, E Holtz, I Karimé, N N Saleh
Index: J. Org. Chem. 66(18) , 6057-63, (2001)
Full Text: HTML
Abstract
The domino C,O-cyclodialkylation reaction of dilithiated 1,3-dicarbonyl compounds with 1,4-dibromo-2-butene resulted in regio- and diastereoselective formation of 2-alkylidene-5-vinyltetrahydrofurans. The cyclization of 1,3-dicarbonyl dianions with 1-bromo-2-chloroethane regio- and diastereoselectively afforded 2-alkylidenetetrahydrofurans under thermodynamic reaction control.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
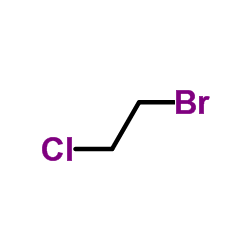 |
1-Bromo-2-chloroethane
CAS:107-04-0 |
C2H4BrCl |
Related Articles:
More...
|
Simultaneous derivatization and solid-based disperser liquid...
2015-03-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 983-984 , 55-61, (2015)] |
|
Efficient synthesis of furan-2-ylacetates, 7-(alkoxycarbonyl...
2005-11-25 [J. Org. Chem. 70 , 10013-10029, (2005)] |
|
Dissociative double ionization of 1-bromo-2-chloroethane irr...
2011-08-14 [J. Chem. Phys. 135(6) , 064303, (2011)] |
|
A conformational study of 1-bromo-2-chloroethane in zeolites...
2009-07-21 [Langmuir 25(14) , 8042-50, (2009)] |
|
Characterization of the genotoxic action of three structural...
1993-02-01 [Mutat. Res. 285(2) , 209-17, (1993)] |