Quaternary ammonium analogs of ether lipids inhibit the activation of protein kinase C and the growth of human leukemia cell lines.
F Civoli, L W Daniel
Index: Cancer Chemother. Pharmacol. 42(4) , 319-26, (1998)
Full Text: HTML
Abstract
ET-18-OCH3 (1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine) is a representative of the first generation of antitumor ether lipids and is a model in the development of new compounds including a series of quaternary ammonium analogs (QAA). In the present study, we evaluated the QAA as inhibitors of cell growth and studied their mechanism of action.We compared the effects of the QAA on the proliferation of human leukemia cell lines which are sensitive (HL-60) or resistant to ET-18-OCH3 (HL-60R and K562). In addition we used cell fractionation and enzymatic assays to determine the effects of QAA on protein kinase C (PKC) translocation in response to 12-O-tetradecanoyl-phorbol-13-acetate (TPA).The QAA and ET-18-OCH3 were approximately equally effective inhibitors of HL-60 cell growth. However, the QAA were more effective inhibitors of K562 and HL-60R cell proliferation. The HL-60R cells, which were selected for resistance to ET-18-OCH3, were also resistant to BM 41.440 which is structurally similar. In serum-free medium, the phosphorus-containing compounds (ET-18-OCH3, BM 41.440 and He-PC) were much more effective inhibitors (8-20-fold) of the K562 cell line while the activities of the QAA were only moderately increased (1.2-2.3-fold). When serum albumin was added to the serum-free medium, the K562 cells became resistant to ET-18-OCH3, suggesting that albumin is responsible for the differential sensitivity. The QAA compounds, which inhibit PKC activity in vitro, inhibited cell proliferation. However, a QAA which did not inhibit PKC did not inhibit cell proliferation. The phorbol ester TPA stimulates PKC translocation and causes HL-60 cell differentiation to adherent macrophage-type cells. The QAA inhibited TPA-induced cell differentiation and PKC translocation indicating that they also inhibit PKC in intact cells.The cellular effects of the QAA appear to be due to inhibition of PKC. In addition, these data indicate that albumin, which is important as a mediator of the uptake of ET-18-OCH3, has only a small effect on the uptake of QAA. Together these data indicate that the QAA are potential anticancer agents, showing a significant ability to inhibit growth of leukemia cell lines that are resistant to ET-18-OCH3.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
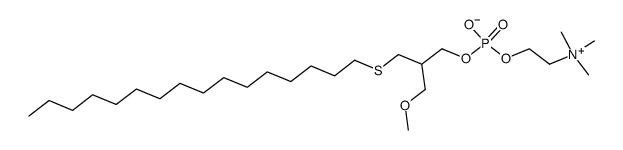 |
ILMOFOSINE
CAS:83519-04-4 |
C26H56NO5PS |
|
Phase I trial of ilmofosine as a 24 hour infusion weekly.
2010-01-01 [Invest. New Drugs 13(3) , 205-10, (1995)] |
|
A phase II trial of ilmofosine in non-small cell bronchogeni...
1996-01-01 [Invest. New Drugs 14(2) , 219-22, (1996)] |
|
Structure-function relationships of alkyl-lysophospholipid a...
1993-06-01 [Lipids 28(6) , 511-6, (1993)] |
|
Expression of two 50 kDa proteins is associated with sensiti...
1997-10-01 [Eur. J. Cancer 33(11) , 1875-80, (1997)] |
|
Cytotoxicity of ether phospholipid BM 41.440 on neuroblastom...
1995-01-01 [J. Cancer Res. Clin. Oncol. 121(5) , 262-6, (1995)] |