Structure-function relationships of alkyl-lysophospholipid analogs in selective antitumor activity.
W R Vogler, A C Olson, J Hajdu, M Shoji, R Raynor, J F Kuo
Index: Lipids 28(6) , 511-6, (1993)
Full Text: HTML
Abstract
This investigation was initiated in order to delineate the structure-function relationship of the anticancer alkyl-lysophospholipids and assess their degree of selective cytotoxicity toward neoplastic cells. A series of glycerol phosphocholine analogs with varying substitutions in the sn-1 and sn-2 position were tested for their inhibitory activity as measured by thymidine incorporation, clonogenic assays and effects on protein kinase C activity against a series of human leukemic cell lines and healthy bone marrow progenitor cells. The IC50 was determined for each of the compounds in each cell line and healthy bone marrow cells following a 4-h incubation. The data indicated that a 16-18 carbon chain at the sn-1 coupled with a short substitution at sn-2 had the broadest antitumor activity and was the least toxic to normal bone marrow cells. The results provide a number of useful leads toward the design and development of potentially more active phospholipid compounds.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
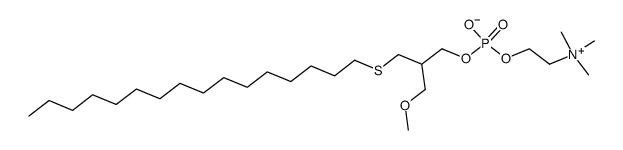 |
ILMOFOSINE
CAS:83519-04-4 |
C26H56NO5PS |
|
Phase I trial of ilmofosine as a 24 hour infusion weekly.
2010-01-01 [Invest. New Drugs 13(3) , 205-10, (1995)] |
|
A phase II trial of ilmofosine in non-small cell bronchogeni...
1996-01-01 [Invest. New Drugs 14(2) , 219-22, (1996)] |
|
Quaternary ammonium analogs of ether lipids inhibit the acti...
1998-01-01 [Cancer Chemother. Pharmacol. 42(4) , 319-26, (1998)] |
|
Expression of two 50 kDa proteins is associated with sensiti...
1997-10-01 [Eur. J. Cancer 33(11) , 1875-80, (1997)] |
|
Cytotoxicity of ether phospholipid BM 41.440 on neuroblastom...
1995-01-01 [J. Cancer Res. Clin. Oncol. 121(5) , 262-6, (1995)] |