| Structure | Name/CAS No. | Articles |
|---|---|---|
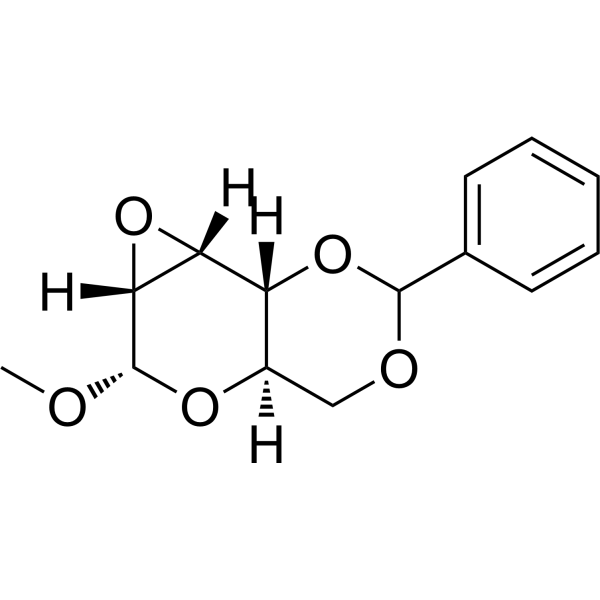 |
METHYL 2,3-ANHYDRO-4,6-O-BENZYLIDENE-α-D-ALLOPYRANOSIDE
CAS:3150-15-0 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
METHYL 2,3-ANHYDRO-4,6-O-BENZYLIDENE-α-D-ALLOPYRANOSIDE
CAS:3150-15-0 |