Antineoplastic agents 500. Narcistatin.
George R Pettit, Noeleen Melody, Michael Simpson, Michael Thompson, Delbert L Herald, John C Knight
Index: J. Nat. Prod. 66(1) , 92-6, (2003)
Full Text: HTML
Abstract
An efficient procedure was found for synthetic conversion of the sparingly soluble anticancer isocarbostyril narciclasine (1), a component of various Narcissus species, to a cyclic phosphate designated narcistatin (3b). The reaction between narciclasine, tetrabutylammonium dihydrogen phosphate, and p-toluenesulfonic acid in pyridine afforded pyridinium narcistatin (3a) in reasonable yields. Transformation of narcistatin (3a) to, for example, the water-soluble prodrug sodium narcistatin (3d) was easily achieved by cation exchange chromatography. Narcistatin (3b) and 15 salt derivatives were evaluated against a panel of human cancer cell lines, and the range (0.1-0.01) of GI(50) values in micro g/mL was found to parallel that shown by the parent narciclasine.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
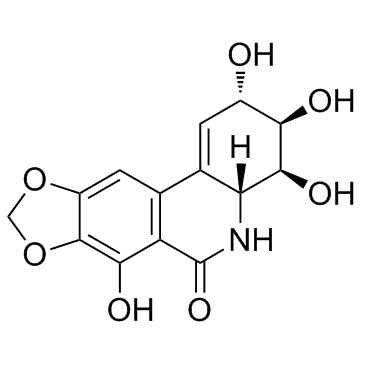 |
Narciclasine
CAS:29477-83-6 |
C14H13NO7 |
|
Simultaneous quantification of Amaryllidaceae alkaloids from...
2012-12-01 [J. Pharm. Biomed. Anal. 71 , 187-92, (2012)] |
|
Seasonal accumulation of major alkaloids in organs of pharma...
2013-04-01 [Phytochemistry 88 , 43-53, (2013)] |
|
[Study on chemical constituents of the bulbs of Lycoris long...
2011-09-01 [Zhong Yao Cai 34(9) , 1366-8, (2011)] |
|
Selective cytochrome P450 3A4 inhibitory activity of Amaryll...
2009-06-15 [Bioorg. Med. Chem. Lett. 19 , 3233-7, (2009)] |
|
Antineoplastic agents. 454. Synthesis of the strong cancer c...
2009-07-01 [J. Nat. Prod. 72(7) , 1279-82, (2009)] |