Conformational landscape of platinum(II)-tetraamine complexes: DFT and NBO studies.
Austin B Yongye, Marc A Giulianotti, Adel Nefzi, Richard A Houghten, Karina Martínez-Mayorga
Index: J. Comput. Aided Mol. Des. 24(3) , 225-35, (2010)
Full Text: HTML
Abstract
The potential energy surfaces of chiral tetraamine Pt(II) coordination complexes were computed at the B3LYP/LANL2DZ level of theory by a systematic variation of two dihedral angles: C12-C15-C34-C37 (theta) and C24-C17-C31-C48 (psi) employing a grid resolution of 30 degrees . Potential energy surfaces calculated using density functional theory methods and Boltzmann-derived populations revealed strong preference for one diasteromer of each series studied. In addition, natural bond orbital analysis show that the minima are stabilized predominantly by a combination of electronic interactions between two phenyl groups, the phenyl groups and the Pt(2+) ion, as well as with the amine groups. Additional experimental characterization of the diasteroisomers studied here is in progress and will permit further molecular modeling studies with the appropriate stereochemistry.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
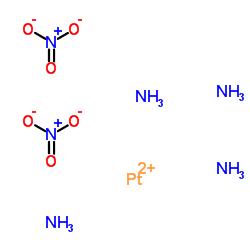 |
Platinum(2+) nitrate ammoniate (1:2:4)
CAS:20634-12-2 |
H12N6O6Pt |
|
Block by ruthenium red of the ryanodine-activated calcium re...
1993-12-01 [J. Gen. Physiol. 102(6) , 1031-56, (1993)] |
|
Symmetry properties of tetraammine platinum(II) with C2v and...
2005-03-01 [J. Zhejiang Univ. Sci. B 6(3) , 222-6, (2005)] |
|
Irradiation enhances cellular uptake of carboplatin.
1995-10-15 [Int. J. Radiat. Oncol. Biol. Phys. 33(3) , 641-6, (1995)] |
|
Irradiation-enhanced binding of carboplatin to DNA.
1995-12-01 [Int. J. Radiat. Biol. 68(6) , 609-14, (1995)] |
|
Synthesis of platinum(II) chiral tetraamine coordination com...
2006-01-01 [J. Comb. Chem. 8(5) , 780-3, (2006)] |