Direct chiral resolution of phenylalkylamines using a crown ether chiral stationary phase.
H Y Aboul-Enein, V Serignese
Index: Biomed. Chromatogr. 11(1) , 7-10, (1997)
Full Text: HTML
Abstract
A direct, isocratic high-performance liquid chromatographic method is described for the enantiomeric resolution of a number of phenylalkylamines, namely, racemic cathinone, amphetamine, norephedrine, and norphenylephrine, without sample derivatization. The separations were achieved on an S-18-crown-6-ether chiral stationary phase known as CR(+). The chromatographic parameters alpha (separation factor) and Rs (resolution factor) lay within a narrow range for all compounds used in this study except for cathinone, which resulted in high alpha and Rs values. The recognition mechanism for this column involves the interaction of the crown structure with a charged primary ammonium ion. The stereochemical structures of the compounds in this study contribute to the results obtained for the chromatographic parameters, especially in cathinone's case. This paper will discuss the recognition mechanism contributing to the high alpha and Rs, values obtained for cathinone.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
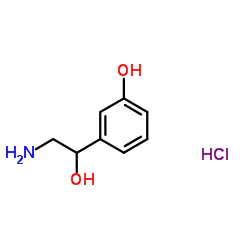 |
Norphenylephrine hydrochloride
CAS:4779-94-6 |
C8H12ClNO2 |
|
Selective synthesis of racemic 1-11C-labelled norepinephrine...
1994-04-01 [Appl. Radiat. Isot. 45(4) , 515-21, (1994)] |
|
Chiral separations on multichannel microfluidic chips.
2005-12-01 [Electrophoresis 26(24) , 4774-9, (2005)] |
|
In vivo alpha(1)-adrenergic lipolytic activity in subcutaneo...
2002-04-01 [J. Pharmacol. Exp. Ther. 301(1) , 229-33, (2002)] |
|
[Therapy of hypotension in pregnancy using norfenefrine hydr...
1992-01-01 [Z. Geburtshilfe Perinatol. 196(1) , 21-5, (1992)] |
|
The effects of m-octopamine on salivary flow rates and prote...
1992-11-01 [Comp. Biochem. Physiol. C, Comp. Pharmacol. Toxicol. 103(3) , 469-76, (1992)] |