Regio- and stereoselectivity in the coupling reaction of secologanin with dopamine derivatives.
G Beke, L F Szabó, B Podányi
Index: J. Nat. Prod. 64(3) , 332-40, (2001)
Full Text: HTML
Abstract
The coupling reaction of tetraacetylsecologanin with dopamine and its N-benzyl derivative was investigated. In both series, stereoisomers at C-1, as well as regioisomer normal and neo compounds, were formed. Moreover, the N-unsubstituted products were partially lactamized, and the N-benzyl derivatives epimerized at C-1. In the products, the R configuration of C-1 over the S and the formation of the normal structure over the neo one predominated. The epimerization of both epimers gave an equilibrium of R and S in a ratio of 7:3 and was interpreted by cleavage of the C-1-N-2 bond. The fact that lactamization was much faster in the R than in the S series was explained on the basis of the supposed transition states. The structure, the configuration of C-1, and in several cases the conformations were established by detailed NMR studies and supported by chemical correlations.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
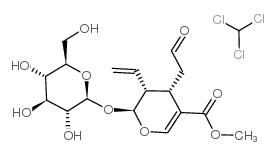 |
Secologanin
CAS:19351-63-4 |
C17H24O10 |
|
The leaf epidermome of Catharanthus roseus reveals its bioch...
2008-03-01 [Plant Cell 20(3) , 524-42, (2008)] |
|
2,4-D and alkaloid accumulation in periwinkle cell suspensio...
1994-01-01 [Biochimie 76(5) , 410-6, (1994)] |
|
Indole alkaloid biosynthesis in Catharanthus roseus: new enz...
2000-12-01 [Plant J. 24 , 797, (2000)] |
|
Strategies for engineering plant natural products: the irido...
2012-01-01 [Meth. Enzymol. 515 , 189-206, (2012)] |
|
Secologanin synthase which catalyzes the oxidative cleavage ...
2000-01-01 [Phytochemistry 53(1) , 7-12, (2000)] |