Investigation of the coupling reaction of tetraacetylsecologanin with oxotryptamine and its derivative.
A Patthy-Lukáts, G Beke, L F Szabó, B Podányi
Index: J. Nat. Prod. 64(8) , 1032-9, (2001)
Full Text: HTML
Abstract
The coupling reaction of tetraacetylsecologanin with 2,3-dihydro-2-oxotryptamine and its N(b)-benzyl derivative was investigated. With the benzylated amine, the reaction was stopped at the tetracyclic ester level, and with the unsubstituted amine it was immediately followed by lactamization. In both cases, the products were formed with high stereoselectivity at C-3, but as an epimeric pair of 7R and 7S in a ratio of 1:3. The bulky benzyl substituent at N-4 directed the stereoselectivity at C-3 in favor of the S configuration. In the nonbenzylated compounds, the reversible coupling reaction is probably nonstereoselective, but in lactamization the 3R epimer is sterically favored and faster and gives the final lactam in this configuration. The formation of the spiro compounds may serve as a model reaction in the interpretation of the stereoselectivity of the coupling reaction of secologanin with tryptamine in the presence of strictosidine synthase.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
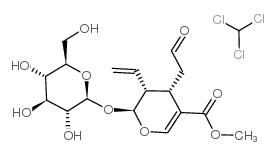 |
Secologanin
CAS:19351-63-4 |
C17H24O10 |
|
The leaf epidermome of Catharanthus roseus reveals its bioch...
2008-03-01 [Plant Cell 20(3) , 524-42, (2008)] |
|
2,4-D and alkaloid accumulation in periwinkle cell suspensio...
1994-01-01 [Biochimie 76(5) , 410-6, (1994)] |
|
Indole alkaloid biosynthesis in Catharanthus roseus: new enz...
2000-12-01 [Plant J. 24 , 797, (2000)] |
|
Strategies for engineering plant natural products: the irido...
2012-01-01 [Meth. Enzymol. 515 , 189-206, (2012)] |
|
Secologanin synthase which catalyzes the oxidative cleavage ...
2000-01-01 [Phytochemistry 53(1) , 7-12, (2000)] |