The crystal structure of the signal recognition particle in complex with its receptor.
Sandro F Ataide, Nikolaus Schmitz, Kuang Shen, Ailong Ke, Shu-ou Shan, Jennifer A Doudna, Nenad Ban
Index: Science 331 , 881-886, (2011)
Full Text: HTML
Abstract
Cotranslational targeting of membrane and secretory proteins is mediated by the universally conserved signal recognition particle (SRP). Together with its receptor (SR), SRP mediates the guanine triphosphate (GTP)-dependent delivery of translating ribosomes bearing signal sequences to translocons on the target membrane. Here, we present the crystal structure of the SRP:SR complex at 3.9 angstrom resolution and biochemical data revealing that the activated SRP:SR guanine triphosphatase (GTPase) complex binds the distal end of the SRP hairpin RNA where GTP hydrolysis is stimulated. Combined with previous findings, these results suggest that the SRP:SR GTPase complex initially assembles at the tetraloop end of the SRP RNA and then relocalizes to the opposite end of the RNA. This rearrangement provides a mechanism for coupling GTP hydrolysis to the handover of cargo to the translocon.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
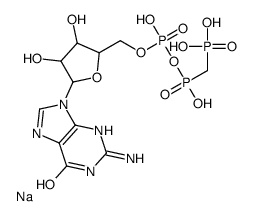 |
GppCp(GMPPCP)
CAS:10470-57-2 |
C11H15N5Na3O13P3 |
|
Polarized endosome dynamics by spindle asymmetry during asym...
2015-12-10 [Nature 528 , 280-5, (2015)] |
|
Dynamic and thermodynamic response of the Ras protein Cdc42H...
2014-10-23 [J. Mol. Biol. 426(21) , 3520-38, (2014)] |
|
Regulation of human tissue transglutaminase function by magn...
1998-01-16 [J. Biol. Chem. 273 , 1776-1781, (1998)] |
|
Functional characterization of solute carrier (SLC) 26/sulfa...
2016-04-01 [Biochim. Biophys. Acta 1858 , 698-705, (2016)] |
|
LRRK2 kinase activity is dependent on LRRK2 GTP binding capa...
2011-01-01 [PLoS ONE 6 , e23207, (2011)] |