Preferential inhibition of 5-trifluoromethyl-2'-deoxyuridine phosphorylation by 5'-amino-5'-deoxythymidine in uninfected versus herpes simplex virus-infected cells.
P H Fischer, D G Murphy, R Kawahara
Index: Mol. Pharmacol. 24(1) , 90-6, (1983)
Full Text: HTML
Abstract
The cytotoxic effects of 5-trifluoromethyl-2'-deoxyuridine (CF3dUrd) were effectively antagonized by 5'-amino-5'-deoxythymidine (5'-AdThd). The antiproliferative actions of CF3dUrd were reduced in a dose-dependent manner by 5'-AdThd in both HeLa and Vero cells. In addition, the ability of CF3dUrd to kill HeLa cells (95% at 1 microM and 99% at 3 microM), as measured by cloning efficiency, was ablated entirely by 5'-AdThd (300 microM). In contrast, the inhibition of herpes simplex virus Type 2 (HSV-2) replication in HeLa cells was not antagonized by 5'-AdThd. In Vero cells, the combination of CF3dUrd and 5'-AdThd produced a greater antiviral effect than either agent alone. The reduction in CF3dUrd cytotoxicity caused by 5'-AdThd in uninfected HeLa and Vero cells was associated with decreased intracellular levels of CF3dUrd nucleotides. In contrast, in HSV-2-infected Vero cells the intracellular levels of CF3dUrd nucleotides were slightly elevated by 5'-AdThd and, in virally infected HeLa cells, a 300-fold excess of 5'-AdThd reduced CF3dUrd uptake only marginally. Since the relative abundance of these phosphorylated derivatives of CF3dUrd was not markedly changed by 5'-AdThd, preferential inhibition of the mammalian thymidine kinase (EC 2.7.1.21) was suggested. Using CF3dUrd as the substrate, the ability of 5'-AdThd to inhibit thymidine kinase activity in extracts prepared from parallel cultures of mock-infected or HSV-2 infected HeLa cells was compared. CF3dUrd was phosphorylated to a lesser extent and the reaction was more potently inhibited by 5'-AdThd in the extracts prepared from the uninfected cells. The HeLa cell and HSV-2 thymidine kinases were purified by affinity column chromatography, and kinetic analyses were then done. Using CF3dUrd as the variable substrate, the Ki values for 5'-AdThd were 2.2 microM for the HeLa enzyme and 36 microM for the viral enzyme. Km values for CF3dUrd were 2.6 microM and 4 microM for the viral and mammalian enzymes, respectively. These data account for the ability of 5'-AdThd to inhibit preferentially the phosphorylation of CF3dUrd in uninfected host cells. The presence of 5'-AdThd substantially increased the therapeutic index of CF3dUrd, indicating that this drug combination, an example of specific inhibition, warrants investigation in vivo.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
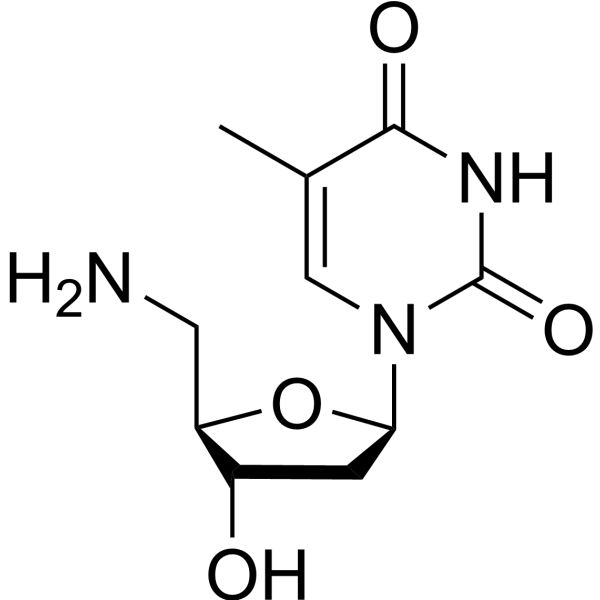 |
Thymidine,5'-amino-5'-deoxy
CAS:25152-20-9 |
C10H15N3O4 |
|
Measurement of carbamoylating activity of nitrosoureas and i...
1986-07-15 [Biochem. Pharmacol. 35(14) , 2359-65, (1986)] |
|
Novel acyclic amide-conjugated nucleosides and their analogu...
2009-02-01 [Nucleosides Nucleotides Nucleic Acids 28 , 103-117, (2009)] |
|
Carbamoylation reactions of N-methyl-N'-aryl-N-nitrosoureas ...
1992-04-01 [Carcinogenesis 13(4) , 699-702, (1992)] |
|
Template-directed addition of nucleosides to DNA by the BfiI...
2008-07-01 [Nucleic Acids Res. 36 , 3969-3977, (2008)] |
|
Modulation of the metabolism and cytotoxicity of iododeoxyur...
1983-05-01 [Mol. Pharmacol. 23(3) , 709-16, (1983)] |