Functionalization of pyrimidine and purine nucleosides at C4 and C6: C-nucleophilic substitution of their C4- and C6-(1,2,4-triazol-1-yl) derivatives.
Victor Timoshchuk
Index: Nucleosides Nucleotides Nucleic Acids 24(5-7) , 1043-6, (2005)
Full Text: HTML
Abstract
A study of C-nucleophilic substitution at the C4-position on pyrimidine and C6-position on 2'-deoxyguanosine to produce novel nucleosides is presented with the spectroscopic properties of their respective substitution products. C4-(1,2,4-triazol-1-yl) pyrimidine nucleosides 1 were treated with nitroalkanes, malononitrile, acetylacetone, ethyl nitroacetate, acetoacetate and cyanoacetate at 100 degrees C in dioxane in the presence of DBU resulting in the production of novel nucleosides 2-11. To explore the application of this methodology to purine chemistry, this approach was used to produce novel analogs from 2'-deoxyguanosine. We found that the triazolo derivative 12 undergoes C-nucleophilic substitution with nitromethane, malononitrile, acetylacetone, ethyl nitroacetate and cyanoacetate in the presence of potassium carbonate (K2CO3) in DMF at 100 degrees C to give novel nucleosides 13-17.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
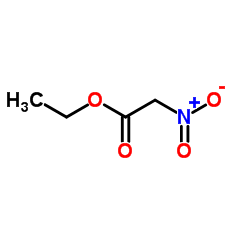 |
ETHYL NITROACETATE
CAS:626-35-7 |
C4H7NO4 |
|
[Experimental establishment of the maximum permissible conce...
1980-07-01 [Gig. Tr. Prof. Zabol. (7) , 49-51, (1980)] |
|
Synthesis and biological evaluation of DL-4,4-difluoroglutam...
1996-01-05 [J. Med. Chem. 39(1) , 66-72, (1996)] |
|
Formation of γ-oxoacids and 1H-pyrrol-2(5H)-ones from α,β-un...
2010-11-05 [J. Org. Chem. 75(21) , 7435-8, (2010)] |
|
Facile synthesis of alpha,alpha-diisobutylglycine and anchor...
2003-12-12 [J. Org. Chem. 68(25) , 9854-7, (2003)] |
|
Acid-base-catalysed condensation reaction in water: isoxazol...
2012-02-13 [Chemistry 18(7) , 2081-93, (2012)] |