Journal of Organic Chemistry
2010-11-05
Formation of γ-oxoacids and 1H-pyrrol-2(5H)-ones from α,β-unsaturated ketones and ethyl nitroacetate.
Maialen Aginagalde, Tamara Bello, Carme Masdeu, Yosu Vara, Ana Arrieta, Fernando P Cossío
Index: J. Org. Chem. 75(21) , 7435-8, (2010)
Full Text: HTML
Abstract
Michael addition of ethyl nitroacetate on α,β-unsaturated ketones followed by Nef oxidation under hydrolytic conditions yields γ-oxoacids instead of the corresponding α,δ-dioxoesters. A concerted decarboxylation step is proposed on the basis of computational results. Finally, conversion of the γ-ketoacids thus prepared into 1H-pyrrol-2(5H)-ones by reaction with primary amines under Paal-Knorr conditions is also reported.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
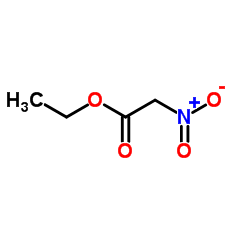 |
ETHYL NITROACETATE
CAS:626-35-7 |
C4H7NO4 |
Related Articles:
More...
|
[Experimental establishment of the maximum permissible conce...
1980-07-01 [Gig. Tr. Prof. Zabol. (7) , 49-51, (1980)] |
|
Synthesis and biological evaluation of DL-4,4-difluoroglutam...
1996-01-05 [J. Med. Chem. 39(1) , 66-72, (1996)] |
|
Facile synthesis of alpha,alpha-diisobutylglycine and anchor...
2003-12-12 [J. Org. Chem. 68(25) , 9854-7, (2003)] |
|
Functionalization of pyrimidine and purine nucleosides at C4...
2005-01-01 [Nucleosides Nucleotides Nucleic Acids 24(5-7) , 1043-6, (2005)] |
|
Acid-base-catalysed condensation reaction in water: isoxazol...
2012-02-13 [Chemistry 18(7) , 2081-93, (2012)] |