Clinical evaluation of hydrocortisone valerate 0.2% ointment.
J Sefton, J S Loder, A A Kyriakopoulos
Index: Clin. Ther. 6(3) , 282-93, (1984)
Full Text: HTML
Abstract
Evaluations of the comparative efficacy and safety of hydrocortisone valerate 0.2% ointment were made in six double-blind, multicenter trials involving a total of 485 patients, 209 with atopic dermatitis and 276 with plaque psoriasis. The vasoconstrictor activity of hydrocortisone valerate 0.2% ointment was also assessed in normal subjects. Hydrocortisone valerate 0.2% ointment displayed therapeutic effects within three days. In terms of global evaluations of efficacy, hydrocortisone valerate was more effective than vehicle and was comparable to other intermediate or moderate potency corticosteroid ointments. The vasoconstrictor activity of hydrocortisone valerate 0.2% ointment was greater than that of other moderate potency ointments.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
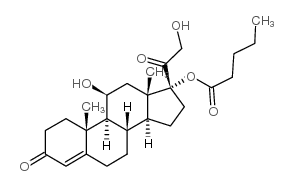 |
Hydrocortisone Valerate
CAS:57524-89-7 |
C26H38O6 |
|
Topical corticosteroid compounding: effects on physicochemic...
1989-11-01 [J. Am. Acad. Dermatol. 21(5 Pt 1) , 979-84, (1989)] |
|
Addition of a topically applied corticosteroid to a modified...
1985-11-01 [J. Am. Acad. Dermatol. 13(5 Pt 1) , 784-91, (1985)] |
|
Temporal infiltration of leukocyte subsets into mouse skin i...
1992-11-01 [Agents Actions 37(3-4) , 260-7, (1992)] |
|
Contact hypersensitivity to tixocortol pivalate.
1998-05-01 [J. Am. Acad. Dermatol. 38(5 Pt 1) , 691-5, (1998)] |
|
A dermatitis-eosinophilia syndrome. Treatment with methylpre...
1984-12-01 [Arch. Dermatol. 120(12) , 1595-7, (1984)] |