Organic Letters
2006-10-12
Concise assembly of the polycyclic frameworks associated with the hapalindole and fischerindole alkaloids.
Martin G Banwell, Xinghua Ma, Rebecca M Taylor, Anthony C Willis
Index: Org. Lett. 8(21) , 4959-61, (2006)
Full Text: HTML
Abstract
[reaction: see text] Reaction of N-methylindole (4) with 6,6-dibromobicyclo[3.1.0]hexane (5) in the presence of silver tetrafluoroborate affords conjugate 7 in 67% yield. This product can be readily elaborated to compounds 12b and 13b which embody the polycyclic frameworks associated with members of the hapalindole and fischerindole classes of alkaloids. The chiral-auxiliary-substituted 6,6-dibromobicyclo[3.1.0]hexanes 21 and 22 react with indole to give adducts likely to be useful in the enantioselective total synthesis of the title alkaloids.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
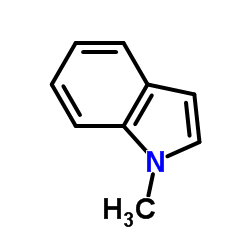 |
1-Methylindole
CAS:603-76-9 |
C9H9N |
Related Articles:
More...
|
Natural compounds boldine and menthol are antagonists of hum...
2014-06-01 [Neurogastroenterol. Motil. 26(6) , 810-20, (2014)] |
|
Degradation of substituted indoles by an indole-degrading me...
1991-09-01 [Appl. Environ. Microbiol. 57(9) , 2622-7, (1991)] |
|
Single-fluorophore-based fluorescent probes enable dual-chan...
2014-08-11 [Anal. Chim. Acta 839 , 74-82, (2014)] |
|
Photodissociation dynamics of N-methylindole, N-methylpyrrol...
2009-04-23 [J. Phys. Chem. A 113(16) , 3881-5, (2009)] |
|
Spectroscopic characterization studies of 1-methyl indole wi...
2010-09-15 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 77(1) , 179-83, (2010)] |