Influence of conformationally constrained amino acids replacing positions 2 and 3 of arginine vasopressin (AVP) and its analogues on their pharmacological properties.
Dariusz Sobolewski, Adam Prahl, Izabela Derdowska, Anna Kwiatkowska, Jirina Slaninová, Bernard Lammek
Index: Protein Pept. Lett. 14(3) , 213-7, (2007)
Full Text: HTML
Abstract
Synthesis of thirteen new analogues of arginine vasopressin (AVP) has been described. Amino acid residues at positions 2 and 3 of AVP, [3-mercaptopropionic acid (Mpa)(1)]AVP (dAVP), [Mpa(1),d-Arg(8)]VP (dDAVP) and [Mpa(1),Val(4),d-Arg(8)]VP (dVDAVP) were replaced with one amino acid residue using sterically constrained non-proteinogenic amino acids, 4-aminobenzoic acid (Abz), cis-4-aminocyclohexanecarboxylic acid (ach) or its trans-isomer (Ach). In the case of a potent V(1a) antagonist, [1-mercaptocyclohexaneacetic acid (Cpa)(1)]AVP, only one similar analogue has been prepared by replacing positions 2 and 3 with Abz. Unfortunately, all new peptides were inactive in bioassays for the pressor, antidiuretic and uterotonic in vitro activities in the rat.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
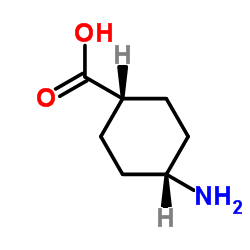 |
4-Aminocyclohexanecarboxylic acid
CAS:3685-23-2 |
C7H13NO2 |
|
Opioid deltorphin C analogues containing cis- or trans-2- or...
1999-01-01 [Arzneimittelforschung 49(1) , 6-12, (1999)] |
|
cis-4-[[[(2-Chloroethyl)nitrosoamino]carbonyl]methylamino] c...
1984-01-01 [J. Med. Chem. 27(1) , 97-9, (1984)] |
|
Hydrogen-bonding patterns in cis-4-ammoniocyclohexanecarboxy...
2004-10-01 [Acta Crystallogr. C 60(Pt 10) , o759-61, (2004)] |
|
Cytotoxic T lymphocyte epitope analogues containing cis- or ...
2002-09-01 [Bioorg. Med. Chem. 10(9) , 3061-6, (2002)] |
|
Thrombin-induced alterations in lung fluid balance in awake ...
1985-07-01 [J. Appl. Physiol. 58(5) , 1421-7, (1985)] |