Cytotoxic T lymphocyte epitope analogues containing cis- or trans-4-aminocyclohexanecarboxylic acid residues.
Mauro Marastoni, Martina Bazzaro, Fabiola Micheletti, Riccardo Gavioli, Roberto Tomatis
Index: Bioorg. Med. Chem. 10(9) , 3061-6, (2002)
Full Text: HTML
Abstract
In order to improve the immunotherapeutical potential of H-Cys-Leu-Gly-Gly-Leu-Leu-Thr-Met-Val-OH (CLG) peptide, an Epstein-Barr virus (EBV) subdominant epitope derived from the membrane protein LMP2, we have synthesized and tested CLG analogues containing cis- and/or trans-4-aminocyclohexanecarboxylic acid (ACCA) replacing Gly-Gly and/or Thr-Met dipeptide units. All pseudopeptides were tested for metabolic stability and for their capacity to bind HLA-A2 molecules and to sensitize target cells to lysis. All new compounds exhibited higher enzymatic resistance compared to the original CLG and some trans-ACCA-derivatives were able to associate HLA-A2 and to efficiently stimulate CTL responses directed against the CLG natural epitope.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
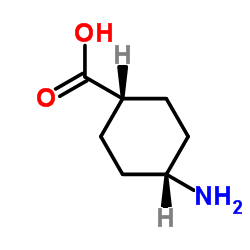 |
4-Aminocyclohexanecarboxylic acid
CAS:3685-23-2 |
C7H13NO2 |
|
Opioid deltorphin C analogues containing cis- or trans-2- or...
1999-01-01 [Arzneimittelforschung 49(1) , 6-12, (1999)] |
|
cis-4-[[[(2-Chloroethyl)nitrosoamino]carbonyl]methylamino] c...
1984-01-01 [J. Med. Chem. 27(1) , 97-9, (1984)] |
|
Hydrogen-bonding patterns in cis-4-ammoniocyclohexanecarboxy...
2004-10-01 [Acta Crystallogr. C 60(Pt 10) , o759-61, (2004)] |
|
Influence of conformationally constrained amino acids replac...
2007-01-01 [Protein Pept. Lett. 14(3) , 213-7, (2007)] |
|
Thrombin-induced alterations in lung fluid balance in awake ...
1985-07-01 [J. Appl. Physiol. 58(5) , 1421-7, (1985)] |