Bioorganic & Medicinal Chemistry Letters
2001-08-06
Synthesis and biological activities of novel structural analogues of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand.
Y Suhara, S Nakane, S Arai, H Takayama, K Waku, Y Ishima, T Sugiura
Index: Bioorg. Med. Chem. Lett. 11(15) , 1985-8, (2001)
Full Text: HTML
Abstract
Novel analogues of 2-arachidonoylglycerol (2-AG), an endogenous cannabinoid receptor ligand, were developed. Chemical synthesis of these analogues (2-AGA105 and 2-AGA109) was accomplished starting from 2-octyn-1-ol and diethyl malonate and employing Wittig coupling of triene phosphonate with an aldehyde intermediate in a convergent and stereoselective manner. These analogues should be useful lead compounds for the development of novel 2-AG mimetics.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
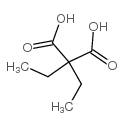 |
diethylmalonic acid
CAS:510-20-3 |
C7H12O4 |
Related Articles:
More...
|
A modified, high yield procedure for the synthesis of unlabe...
1981-01-01 [Prep. Biochem. 11(3) , 339-50, (1981)] |
|
Evaluation of malonanilides as new stabilizers for double-ba...
2000-04-28 [J. Hazard. Mater. 73(3) , 237-44, (2000)] |
|
A general and mild copper-catalyzed arylation of diethyl mal...
2002-01-24 [Org. Lett. 4(2) , 269-72, (2002)] |
|
Effect of various growth conditions on spore formation and b...
1986-03-01 [Can. J. Microbiol. 32(3) , 254-8, (1986)] |
|
Effects of diethylmalonate & alpha-picolinic acid on levels ...
1983-02-01 [Indian J. Biochem. Biophys. 20(1) , 39-42, (1983)] |