The pleckstrin homology domain of Gab-2 is required for optimal interleukin-3 signalsome-mediated responses.
Christine E Edmead, Bridget C Fox, Catherine Stace, Nicholas Ktistakis, Melanie J Welham
Index: Cell. Signal. 18(8) , 1147-55, (2006)
Full Text: HTML
Abstract
The adaptor protein Gab-2 coordinates the assembly of the IL-3 signalsome comprising Gab-2, Grb2, Shc, SHP-2 and PI3K. To investigate the role of the pleckstrin homology domain of Gab-2 in this process, epitope-tagged wild type Gab-2 (WTGab-2), Gab-2 lacking its PH domain (DeltaPHGab-2) and the Gab-2 PH domain alone (PHGab-2) were inducibly expressed in IL-3-dependent BaF/3 cells. Expression of DeltaPHGab-2 reduced IL-3-dependent proliferation and long-term activation of ERK1 and 2 and PKB by IL-3. While we demonstrate that the Gab-2 PH domain can bind PI(3,4,5)P3, it is dispensable for Gab-2 membrane localisation, tyrosine phosphorylation and signalsome formation. Rather, the proline-rich motifs of Gab-2 appear to contribute to the constitutive membrane localisation we observe, independently of tyrosine phosphorylation or the PH domain. Taken together, these findings suggest that once Gab-2 is tyrosine phosphorylated its PH domain is required for the optimal stabilisation of the signalsome, enabling full activation of downstream signals.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
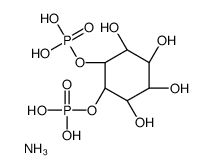 |
D-MYO-INOSITOL 4,5-BIS-PHOSPHATE AMMONIUM SALT
CAS:69256-54-8 |
C6H17NO12P2 |
|
Antigen-induced Ca2+ mobilization in RBL-2H3 cells: role of ...
2005-12-01 [Cell Calcium 38(6) , 581-92, (2005)] |
|
Evidence for lithium-sensitive inositol 4,5-bisphosphate acc...
1992-10-15 [Biochem. J. 287 ( Pt 2) , 437-42, (1992)] |
|
Action and formation of inositol bisphosphate and inositol t...
1990-10-01 [Acta Endocrinol. 123(4) , 459-63, (1990)] |
|
Pathways of dephosphorylation of 1-D-myo-inositol 1,4,5-tris...
1993-07-28 [Biochim. Biophys. Acta 1178(1) , 63-72, (1993)] |
|
P2Y(2) receptor-stimulated phosphoinositide hydrolysis and C...
2000-08-01 [Am. J. Physiol. Lung Cell. Mol. Physiol. 279(2) , L235-41, (2000)] |