Somatostatin inhibition of phosphoinositides turnover in isolated rat acinar pancreatic cells: interaction with bombesin.
C Linard, F Reyl-Desmars, M J Lewin
Index: Regul. Pept. 41(3) , 219-26, (1992)
Full Text: HTML
Abstract
The effects of somatostatin-14 and bombesin on [3H]inositol phosphate accumulation were studied in 24 h myo-[3H]inositol-prelabeled cultured rat acinar cells. Bombesin, 10 nM, stimulated basal formation of phosphatidyl monophosphate (InsP1), phosphatidyl 4,5-biphosphate (InsP2) and inositol 1,4,5-triphosphate (InsP3) by 128 +/- 5.2%, 147 +/- 10% and 155 +/- 5%, respectively. At 5 s, the ED50 value for InsP3 stimulation was 0.70 +/- 0.2 nM. This stimulation was partly blocked (64 +/- 0.04% inhibition) by 10 ng/ml Bordetella pertussis toxin. In contrast to bombesin, somatostatin, 10 nM, inhibited basal InsP1, InsP2 and InsP3 formation. At 5 s, the inhibition degree for InsP3 was 18 +/- 2.5% and the IC50s values 1 +/- 0.09 nM, 1 +/- 0.12 nM and 0.07 +/- 0.005 nM for InsP1, InsP2 and InsP3, respectively. Bombesin-stimulated InsP3 formation was also inhibited by somatostatin. At 5 s, the inhibition degree was 85 +/- 3.5% at 10 nM and the IC50 value, 0.10 +/- 0.05 nM. Furthermore, somatostatin inhibition of bombesin stimulation was partly blocked (66 +/- 4% inhibition) by Bordetella pertussis toxin. These data therefore suggest that the acinar pancreatic cells contain a somatostatin receptor exerting a negative control on basal and bombesin receptor-stimulated phosphatidyl inositol turnover.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
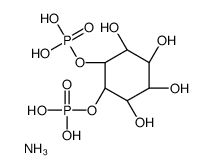 |
D-MYO-INOSITOL 4,5-BIS-PHOSPHATE AMMONIUM SALT
CAS:69256-54-8 |
C6H17NO12P2 |
|
Antigen-induced Ca2+ mobilization in RBL-2H3 cells: role of ...
2005-12-01 [Cell Calcium 38(6) , 581-92, (2005)] |
|
Evidence for lithium-sensitive inositol 4,5-bisphosphate acc...
1992-10-15 [Biochem. J. 287 ( Pt 2) , 437-42, (1992)] |
|
Action and formation of inositol bisphosphate and inositol t...
1990-10-01 [Acta Endocrinol. 123(4) , 459-63, (1990)] |
|
Pathways of dephosphorylation of 1-D-myo-inositol 1,4,5-tris...
1993-07-28 [Biochim. Biophys. Acta 1178(1) , 63-72, (1993)] |
|
P2Y(2) receptor-stimulated phosphoinositide hydrolysis and C...
2000-08-01 [Am. J. Physiol. Lung Cell. Mol. Physiol. 279(2) , L235-41, (2000)] |