The antilipolytic effect of insulin in human adipocytes requires activation of the phosphodiesterase.
P Lönnroth, U Smith
Index: Biochem. Biophys. Res. Commun. 141(3) , 1157-61, (1986)
Full Text: HTML
Abstract
Human fat cells were incubated with two different cAMP analogues, 8-bromocAMP and 6N-monobutyrylcAMP. The former analogue is an excellent substrate for the phosphodiesterase while the latter is resistant to hydrolysis. In the presence of adenosine deaminase, isoproterenol (10(-6)M) stimulated lipolysis 8-10 fold which was similar to the effect exerted by the cAMP analogues. Basal lipolysis and lipolysis activated by 6N-monobutyrylcAMP was not inhibited by insulin even at high concentrations, whereas the effect of 8-bromocAMP was virtually completely inhibited. This effect of insulin was completely prevented by the addition of IBMX. Thus, activation of phosphodiesterase by insulin is necessary to elicit the antilipolytic effect in human adipocytes.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
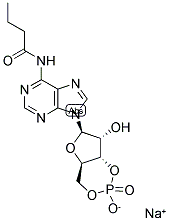 |
N6-Monobutyryladenosine 3′:5′-cyclic monophosphate sodium salt
CAS:70253-67-7 |
C14H17N5NaO7P |
|
Protein kinase A and B-Raf mediate extracellular signal-regu...
2009-11-01 [Mol. Pharmacol. 76 , 1123-1129, (2009)] |
|
Cryptic receptors for insulin-like growth factor II in the p...
1996-06-13 [Biochim. Biophys. Acta 1282(1) , 57-62, (1996)] |
|
Expression of aquaporin 8 and its up-regulation by cyclic ad...
2003-04-01 [Am. J. Obstet. Gynecol. 188(4) , 997-1001, (2003)] |
|
Abnormal adenosine 3'.5'-monophosphate stimulation of renal ...
1989-03-01 [Endocrinology 124(3) , 1184-9, (1989)] |
|
Acquisition of a transcriptionally permissive state during t...
1992-02-01 [Dev. Biol. 149(2) , 457-62, (1992)] |