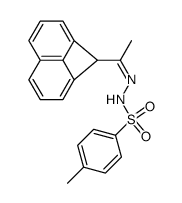
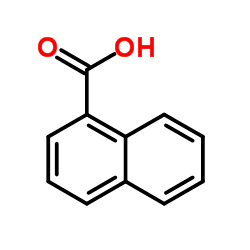
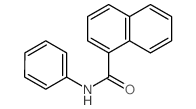
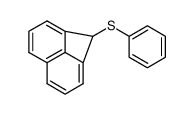
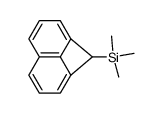
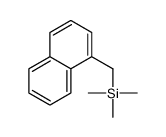
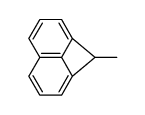
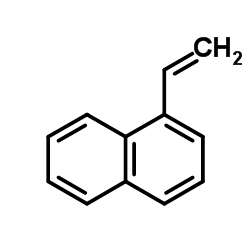
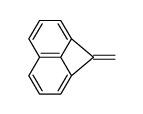
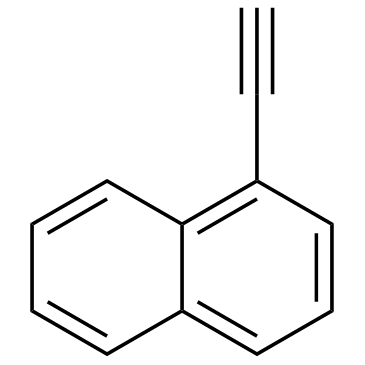
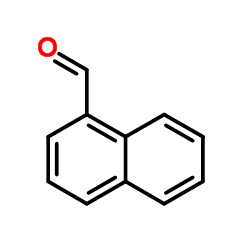
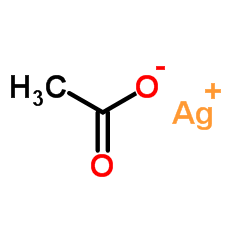
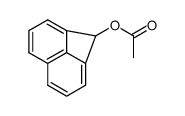
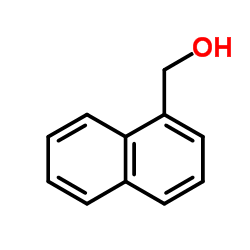
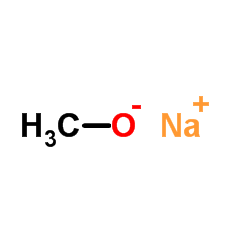
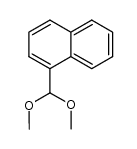
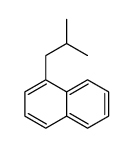
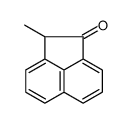
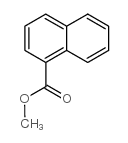
|
~95% |
|
~95% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~44% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~90% |
|
~81% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~82% |
|
~80% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~63% |
|
~% |
|
~63% |
|
~% |
|
~97% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~69% |
|
~60% |
|
~0%
Detail
|
|
~% |
|
~% |
|
~% |
|
~% |
|
~90% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~66% |
|
~% |
|
~% |
|
~% |
![(1H-cyclobuta[de]naphthalen-1-yl)lithium Structure](https://image.chemsrc.com/caspic/426/85924-73-8.png)
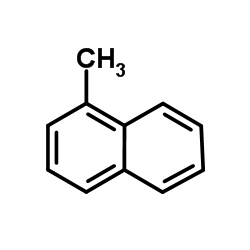
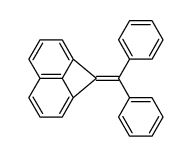
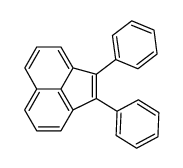
![1-bromo-1H-cyclobuta[de]naphthalene Structure](https://image.chemsrc.com/caspic/087/54125-11-0.png)
![1H-Cyclobuta[de]naphthalene Structure](https://image.chemsrc.com/caspic/242/24973-91-9.png)
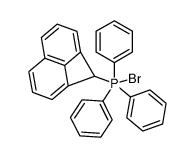
![(1H-cyclobuta[de]naphthalen-1-yl)magnesium bromide Structure](https://image.chemsrc.com/caspic/202/85864-98-8.png)