| Structure | Name/CAS No. | Articles |
|---|---|---|
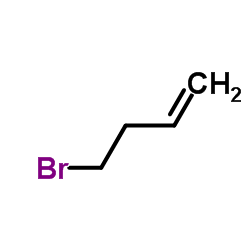 |
4-Brombut-1-en
CAS:5162-44-7 |
Yongcheng Ying, Kanchan Taori, Hyoungsu Kim, Jiyong Hong, Hendrik Luesch
Index: J. Am. Chem. Soc. 130(26) , 8455-9, (2008)
Full Text: HTML
Full details of the concise and convergent synthesis (eight steps, 19% overall yield), its extension to the preparation of a series of key analogues, and the molecular target and pharmacophore of largazole are described. Central to the synthesis of largazole is a macrocyclization reaction for formation of the strained 16-membered depsipeptide core followed by an olefin cross-metathesis reaction for installation of the thioester. The biological evaluation of largazole and its key analogues, including an acetyl analogue, a thiol analogue, and a hydroxyl analogue, suggested that histone deacetylases (HDACs) are molecular targets of largazole and largazole is a class I HDAC inhibitor. In addition, structure-activity relationship (SAR) studies revealed that the thiol group is the pharmacophore of the natural product. Largazole's HDAC inhibitory activity correlates with its antiproliferative activity.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
4-Brombut-1-en
CAS:5162-44-7 |
C4H7Br |
|
[Tetrahedron 60 , 5613, (2004)] |
|
[Synthesis , 3490, (2006)] |
|
Stereoselective epoxidation of 4-bromo-1-butene and 3-butene...
[Biocatal. Biotransformation 1(4) , 283-292, (1988)] |
|
Synthesis and photopolymerization of a liquid-crystalline di...
[Macromolecules 26(6) , 1244-47, (1993)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2024 ChemSrc All Rights Reserved