Journal of the American Chemical Society
2009-07-29
Palladium-catalyzed enantioselective alpha-arylation and alpha-vinylation of oxindoles facilitated by an axially chiral P-stereogenic ligand.
Alexander M Taylor, Ryan A Altman, Stephen L Buchwald
Index: J. Am. Chem. Soc. 131(29) , 9900-1, (2009)
Full Text: HTML
Abstract
The enantioselective alpha-arylation and alpha-vinylation of oxindoles catalyzed by Pd and a biarylmonophosphine ligand with both axial and phosphorus-based chirogenicity is reported. The resultant quaternary carbon stereocenters are formed in high enantiomeric excess, and the conditions tolerate a range of substitution on both the oxindole and the aryl/vinyl coupling partners.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
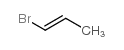 |
1-bromo-1-propene
CAS:590-15-8 |
C3H5Br |
Related Articles:
More...
|
Preparation of (+)-trans-isoalliin and its isomers by chemic...
2014-09-01 [J. Biomol. Tech. 25(3) , 67-76, (2014)] |
|
Structure Analysis of Poly (propylene-β-d oxide)...
[Macromolecules 6(3) , 459-465, (1973)] |
|
cis-trans Isomerization of 1-Bromo-1-propene a...
[J. Am. Chem. Soc. 77(6) , 1682-1684, (1955)] |
|
Substituted Dienols from Palladium Catalyzed Coupling of Hyd...
[Lett. Org. Chem. 5(3) , 158-164, (2008)] |
|
Facile synthesis of trans-S-1-propenyl-L-cysteine sul...
[Bull. Korean Chem. Soc. 32 , 319-320, (2011)] |