RAM/Fam103a1 is required for mRNA cap methylation.
Thomas Gonatopoulos-Pournatzis, Sianadh Dunn, Rebecca Bounds, Victoria H Cowling
Index: Mol. Cell. 44(4) , 585-96, (2011)
Full Text: HTML
Abstract
The 7-methylguanosine cap added to the 5' end of mRNA is required for efficient gene expression in eukaryotes. In mammals, methylation of the guanosine cap is catalyzed by RNMT (RNA guanine-7 methyltransferase), an enzyme previously thought to function as a monomer. We have identified an obligate component of the mammalian cap methyltransferase, RAM (RNMT-Activating Mini protein)/Fam103a1, a previously uncharacterized protein. RAM consists of an N-terminal RNMT-activating domain and a C-terminal RNA-binding domain. As monomers RNMT and RAM have a relatively weak affinity for RNA; however, together their RNA affinity is significantly increased. RAM is required for efficient cap methylation in vitro and in vivo, and is indirectly required to maintain mRNA expression levels, for mRNA translation and for cell viability. Our findings demonstrate that RAM is an essential component of the core gene expression machinery.Copyright © 2011 Elsevier Inc. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
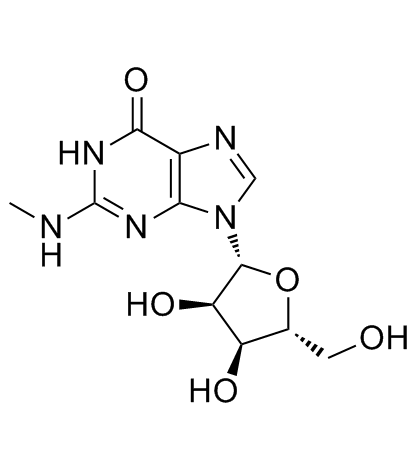 |
N-Methylguanosine
CAS:2140-77-4 |
C11H15N5O5 |
|
Exometabolom analysis of breast cancer cell lines: Metabolic...
2015-01-01 [Sci. Rep. 5 , 13374, (2015)] |
|
Artificial receptors for the extraction of nucleoside metabo...
2014-10-01 [J. Chromatogr. A. 1365 , 12-8, (2014)] |
|
Functional screen reveals SARS coronavirus nonstructural pro...
2009-03-03 [Proc. Natl. Acad. Sci. U. S. A. 106 , 3484-3489, (2009)] |
|
Cap-binding complex (CBC).
2014-01-15 [Biochem. J. 457(2) , 231-42, (2014)] |
|
Identification of a quality-control mechanism for mRNA 5'-en...
2010-09-30 [Nature 467(7315) , 608-11, (2010)] |