1-[[(3-hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties.
Edwin B Villhauer, John A Brinkman, Goli B Naderi, Bryan F Burkey, Beth E Dunning, Kapa Prasad, Bonnie L Mangold, Mary E Russell, Thomas E Hughes
Index: J. Med. Chem. 46 , 2774-2789, (2003)
Full Text: HTML
Abstract
Dipeptidyl peptidase IV (DPP-IV) inhibition has the potential to become a valuable therapy for type 2 diabetes. The synthesis and structure-activity relationship of a new DPP-IV inhibitor class, N-substituted-glycyl-2-cyanopyrrolidines, are described as well as the path that led from clinical development compound 1-[2-[5-cyanopyridin-2-yl)amino]ethylamino]acetyl-2-cyano-(S)-pyrrolidine (NVP-DPP728, 8c) to its follow-up, 1-[[(3-hydroxy-1-adamantyl) amino]acetyl]-2-cyano-(S)-pyrrolidine (NVP-LAF237, 12j). The pharmacological profile of 12j in obese Zucker fa/fa rats along with pharmacokinetic profile comparison of 8c and 12j in normal cynomolgus monkeys is discussed. The results suggest that 12j is a potent, stable, selective DPP-IV inhibitor possessing excellent oral bioavailability and potent antihyperglycemic activity with potential for once-a-day administration.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
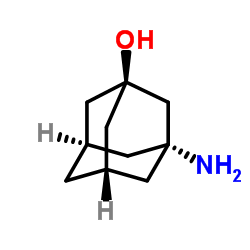 |
3-Amino-1-hydroxyadamantane
CAS:702-82-9 |
C10H17NO |
|
Adamantyl analogues of paracetamol as potent analgesic drugs...
2014-01-01 [PLoS ONE 9(12) , e113841, (2014)] |
|
Liquid chromatographic methods for the determination of vild...
2011-08-15 [Int. J. Biomed. Sci. 7(3) , 201-8, (2011)] |
|
Further calculations on solubility of 3-amino-1-adamantanol ...
[J. Mol. Liq. 219 , 211-215, (2016)] |
|
A Facile and Economical Method to Synthesize Vildagliptin. D...
[Lett. Org. Chem. 11(10) , 780-784, (2014)] |
|
NOVEL TRICYCLIC CYANOPYRROLIDINE DERIVATIVES AS DPP4 INHIBIT...
[Rasayan J. Chem. 6(3) , 230-237, (2013)] |