[Ala1,3,11,15]endothelin-1 analogs with ETB agonistic activity.
T Saeki, M Ihara, T Fukuroda, M Yamagiwa, M Yano
Index: Biochem. Biophys. Res. Commun. 179(1) , 286-92, (1991)
Full Text: HTML
Abstract
A linear peptide analog of endothelin (ET)-1, [Ala1,3,11,15]ET-1 (4AlaET-1), and its truncated peptide analogs were synthesized to study the structural requirements of ET-1 for the recognition of ETs-nonselective ETB receptors. ET-1 exhibited sub-nanomolar binding to two distinct ET receptor subtypes (ETA and ETB), but 4AlaET-1 bound to ETB with an affinity 1,700 times higher than that seen during binding to ETA. The truncated linear peptides 4AlaET-1(6-21), 4AlaET-1(8-21) and N-acetyl-4AlaET-1(10-21) still had high affinity for ETB, whereas 4AlaET-1(6-20) and 4AlaET-1(11-21) displayed remarkably reduced affinity for ETB. Therefore, ET-1 requires the Glu10-Trp21 sequence for ETB binding, but not the disulfide bridges. These ETB-binding peptides elicit endothelium-dependent vasorelaxation of porcine pulmonary arteries in parallel with the binding affinity for ETB, suggesting that they are ETB agonists.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
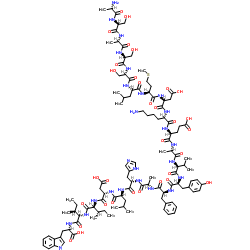 |
(Ala1.3.11.15)-Endothelin-1 trifluoroacetate salt
CAS:121204-87-3 |
C109H163N25O32S |
|
Alterations in ET-1, not nitric oxide, in 1-week-old lambs w...
2003-02-01 [Am. J. Physiol. Heart Circ. Physiol. 284(2) , H480-90, (2003)] |
|
Endothelin receptor blockade does not alter the increase in ...
1996-07-01 [Pediatr. Res. 40(1) , 152-7, (1996)] |
|
Production of monocyte chemoattractant protein-1 and cytokin...
2007-08-06 [Neuroreport 18(12) , 1275-9, (2007)] |
|
Endothelin and structurally related analogs distinguish betw...
1992-03-16 [Biochem. Biophys. Res. Commun. 183(2) , 566-71, (1992)] |
|
Endothelinb receptor agonists produce pulmonary vasodilation...
1995-02-01 [J. Cardiovasc. Pharmacol. 25(2) , 207-15, (1995)] |