Comparison of epidural butamben to celiac plexus neurolytic block for the treatment of the pain of pancreatic cancer.
M Shulman, J E Harris, T R Lubenow, H A Nath, A D Ivankovich
Index: Clin. J. Pain 16(4) , 304-9, (2000)
Full Text: HTML
Abstract
To compare pain relief in metastatic pancreatic cancer patients between neurolytic celiac plexus block (NCPB) and epidural 5% butamben suspension (EBS), a material-based delivery system of a local anesthetic that produces a long-lasting differential nerve block.Open-label patient-selected parallel groups.Urban tertiary care medical center.Twenty-four adult patients with metastatic pancreatic cancer experiencing pain uncontrolled by systemic opioids who were referred to a multidisciplinary pain clinic for interventional therapy.Antecrural NCPB-block with ethanol and epidural 5% butamben suspension injections.Subjective global pain relief assessments on a 0-100% scale were made weekly for 4 weeks and then monthly. Change in opioid use postintervention.Eight patients had a single NCPB and three patients had two NCPB. Four of the former and two of the latter had successful pain relief defined to be a more than 75% reduction in pain when compared with pretreatment maintained for more than 4 weeks or until death (if less than 4 weeks). Thirteen patients received EBS in divided doses. Eleven patients received a cumulative EBS dose of 5 grams, one patient received a cumulative EBS dose of 2.5 grams, and one patient received a cumulative EBS dose of 8.75 grams. Nine of the eleven patients and each of the other two patients had successful pain relief. The overall incidence (85% EBS vs. 55% NCPB), the duration of successful pain relief, and the percent reduction in opioid use did not differ between the two groups. There were no serious complications.EBS appears to be a safe and effective alternative to NCPB in the treatment of pancreatic cancer pain.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
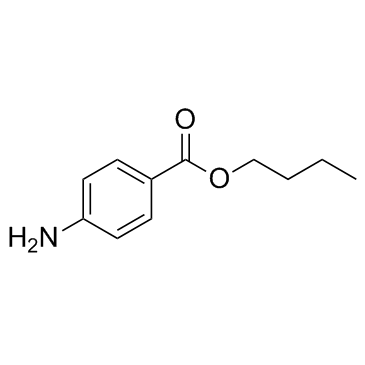 |
butamben
CAS:94-25-7 |
C11H15NO2 |
|
Inhibition of sensory neuronal TRPs contributes to anti-noci...
2012-01-11 [Neurosci. Lett. 506(2) , 297-302, (2012)] |
|
Butamben derivatives enhance BMP-2-stimulated commitment of ...
2011-12-15 [Bioorg. Med. Chem. Lett. 21 , 7363-6, (2011)] |
|
Benzoate X receptors alpha and beta are pharmacologically di...
2002-11-15 [J. Biol. Chem. 277(46) , 43691-7, (2002)] |
|
Nerve blocks with 5% butamben suspension for the treatment o...
1998-01-01 [Reg. Anesth. Pain Med. 23(4) , 395-401, (1998)] |
|
Analgesic synergy between topical morphine and butamben in m...
2003-10-01 [Anesth. Analg. 97(4) , 1103-7, table of contents, (2003)] |