| Structure | Name/CAS No. | Articles |
|---|---|---|
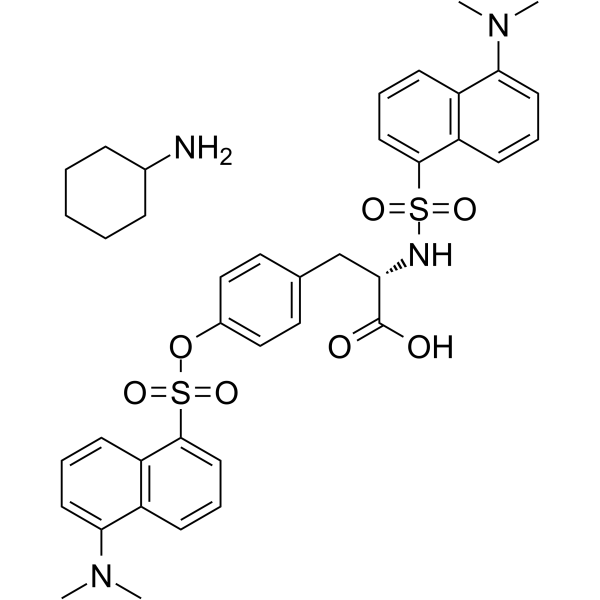 |
N,O-Didansyl-L-tyrosine cyclohexylammonium salt
CAS:102783-47-1 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
N,O-Didansyl-L-tyrosine cyclohexylammonium salt
CAS:102783-47-1 |