| Structure | Name/CAS No. | Articles |
|---|---|---|
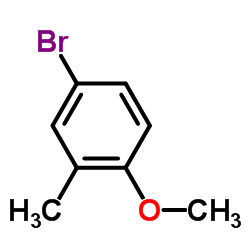 |
4-Bromo-1-methoxy-2-methylbenzene
CAS:14804-31-0 |
James R Vyvyan, Celeste Loitz, Ryan E Looper, Cheryl S Mattingly, Emily A Peterson, Steven T Staben
Index: J. Org. Chem. 69(7) , 2461-8, (2004)
Full Text: HTML
Aromatic bisabolene derivatives were prepared by two methods involving cross-coupling of organozinc reagents. The first synthesis of (+/-)-glandulone A (10), as well as syntheses of (+/-)-curcuhydroquinone (8) and (+/-)-curcuquinone (9), were accomplished via coupling of a secondary alkyl zinc reagent (1,5-dimethyl-4-hexenylzinc halide, 18) to protected bromohydroquinones using Pd(dppf)Cl(2) as catalyst. Coupling of arylzinc halides with alkenyl triflate 16 using Pd(PPh(3))(4) catalyst provided a number of bisabolene derivatives and led to syntheses of dehydro-alpha-curcumene (2), (+/-)-curcuphenol (3), and (+/-)-elvirol (13). A high-yield synthesis of the (+/-)-heliannuol D precursor 29 is also reported using this method.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
4-Bromo-1-methoxy-2-methylbenzene
CAS:14804-31-0 |
C8H9BrO |
|
A useful Pd-catalyzed Negishi coupling approach to benzylic ...
2008-06-19 [Org. Lett. 10(12) , 2517-20, (2008)] |
|
Total synthesis of (±)-heliannuol D, an allelochemical from ...
[Tetrahedron Lett. 41(8) , 1151-1154, (2000)] |
|
(Bmim) Br3 as a New Reagent for Regioselective Monobrominati...
[Chin. J. Chem. 23(11) , 1537-1540, (2005)] |
|
The isopropyl cresols. Carpenter MS and Easter WM.
[J. Org. Chem. 20(4) , 401-411, (1955)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved