Organic Letters
2014-03-21
Visible light photocatalyzed direct conversion of aryl-/heteroarylamines to selenides at room temperature.
Debasish Kundu, Sabir Ahammed, Brindaban C Ranu
Index: Org. Lett. 16(6) , 1814-7, (2014)
Full Text: HTML
Abstract
A novel strategy for the direct conversion of aryl- and heteroarylamines to selenides has been developed via diazotization of amines with tert-butyl nitrite in neutral medium followed by reaction with diaryl/diheteroaryl/dialkyl diselenides in one pot under photocatalysis at room temperature in the absence of any metal. This reaction is also applied for the synthesis of tellurides. The selenylation of heteroarylamine by this protocol is of much significance because of the difficulty in diazotization of these molecules by a standard diazotization method in acid medium.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
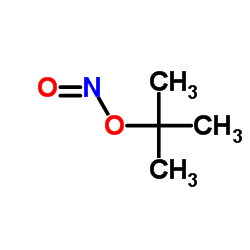 |
t-butyl nitrite
CAS:540-80-7 |
C4H9NO2 |
Related Articles:
More...
|
The convenient preparation of stable aryl-coated zerovalent ...
2015-01-01 [Beilstein J. Nanotechnol. 6 , 1192-8, (2015)] |
|
Gas-phase tyrosine-to-cysteine radical migration in model sy...
2015-01-01 [Eur. J. Mass Spectrom. (Chichester, Eng.) 21 , 589-97, (2015)] |
|
Chemoselective nitration of aromatic sulfonamides with tert-...
2013-01-18 [Chem. Commun. (Camb.) 49(5) , 514-6, (2013)] |
|
Efficient conversion of aromatic amines into azides: a one-p...
2007-04-26 [Org. Lett. 9 , 1809, (2007)] |
|
Aerobic radical multifunctionalization of alkenes using tert...
2013-01-01 [Beilstein J. Org. Chem. 9 , 1713-7, (2013)] |