Strong carbon-surface dative bond formation by tert-butyl isocyanide on the Ge(100)-2 × 1 surface.
Bonggeun Shong, Keith T Wong, Stacey F Bent
Index: J. Am. Chem. Soc. 136(16) , 5848-51, (2014)
Full Text: HTML
Abstract
Carbon dative bond formation between an organic molecule and a semiconductor surface is reported here for the first time. Our studies show that the adsorption of tert-butyl isocyanide on the (100) surface of germanium, measured using Fourier transform infrared spectroscopy, temperature-programmed desorption, and density functional theory calculations, occurs via formation of a dative bond to the surface through the isocyanide carbon. The experimentally observed adsorption energy of 26.8 kcal/mol is the largest among any organic molecule dative bonded on the Ge(100)-2 × 1 surface studied to date. The dative-bonded adsorbate is characterized by a N≡C stretching frequency significantly blue-shifted from that of the free molecule. Moreover, the adsorbate N≡C vibrational frequency red-shifts back toward that of the free molecule upon increasing coverage. These spectroscopic effects are attributed to σ-donation of the isocyanide lone pair electrons to the surface.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
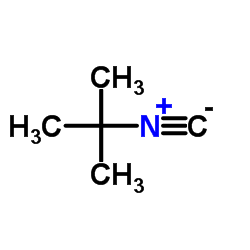 |
Tert-Butyl isocyanide
CAS:7188-38-7 |
C5H9N |
|
Optimization of the Ugi reaction using parallel synthesis an...
2008-01-01 [J. Vis. Exp. (21) , doi:10.3791/942, (2008)] |
|
Palladium-catalyzed synthesis of isocoumarins and phthalides...
2012-11-16 [J. Org. Chem. 77(22) , 10321-8, (2012)] |
|
Towards molecular diversity: dealkylation of tert-butyl amin...
2010-08-21 [Org. Biomol. Chem. 8(16) , 3631-4, (2010)] |
|
Structural and spectroscopic studies of some copper(I) halid...
2008-04-07 [Dalton Trans. (13) , 1710-20, (2008)] |
|
Synthesis and dynamic NMR study of ketenimines derived from ...
2004-01-01 [Mol. Divers. 8(4) , 431-5, (2004)] |