Light-directed generation of the actin-activated ATPase activity of caged heavy meromyosin.
G Marriott, M Heidecker
Index: Biochemistry 35(10) , 3170-4, (1996)
Full Text: HTML
Abstract
An understanding of the molecular mechanism of muscle contraction will require a complete description of the kinetics of the myosin motor in vitro and in vivo. To this end chemical relaxation studies employing light-directed generation of ATP from caged ATP have provided detailed kinetic information in muscle fibers. A more direct approach would be to trigger the actin-activated ATPase activity from a caged myosin, i.e., myosin whose activity is blocked upon derivatization with a photolabile protection group. Herein we report that a new type of caged reagent can be used to prepare a caged heavy meromyosin by modification of critical thiol groups, i.e., a chemically modified motor without activity that can be reactivated at will using a pulse of near-ultraviolet light. Heavy meromyosin modified at Cys-707 with the thiol reactive reagent 1-(bromomethyl)-2-nitro-4,5-dimethoxybenzene does not exhibit an actin-activated ATPase activity and may be viewed as a caged protein. Absorption spectroscopy showed that the thioether bond linking the cage group to Cys-707 is cleaved following irradiation (340-400 nm) via a transient aci-nitro intermediate which has an absorption maximum at 440 nm and decays with a rate constant of 45.6 s(-1). The in vitro motility assay showed that caged heavy meromyosin cannot generate the force necessary to move actin filaments although following irradiation of the image field with a 30 ms pulse of 340-400 nm light the caged group was removed with the concomitant movement of most filaments at a velocity of 0.5-2 micron/s compared to 3-4 micron/s for unmodified HMM. The specificity and simplicity of labeling myosin with the caged reagent should prove useful in studies of muscle contraction in vivo.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
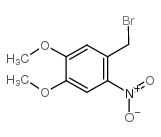 |
1-(BROMOMETHYL)-4,5-DIMETHOXY-2-NITROBENZENE
CAS:53413-67-5 |
C9H10BrNO4 |
|
Caged vanilloid ligands for activation of TRPV1 receptors by...
2006-04-18 [Biochemistry 45(15) , 4915-26, (2006)] |
|
Bright building blocks for chemical biology.
2014-04-18 [ACS Chem. Biol. 9(4) , 855-66, (2014)] |
|
Spatially discrete, light-driven protein expression.
2002-12-01 [Chem. Biol. 9(12) , 1347-53, (2002)] |
|
Model compounds of caged capsaicin: design, synthesis, and p...
2003-11-14 [J. Org. Chem. 68(23) , 9100-4, (2003)] |
|
Light-mediated in cell downregulation of G-quadruplex-contai...
2013-10-04 [Chem. Commun. (Camb.) 49(76) , 8453-5, (2013)] |