| Structure | Name/CAS No. | Articles |
|---|---|---|
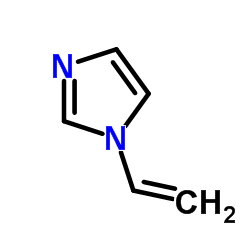 |
Vinylimidazole
CAS:1072-63-5 |
|
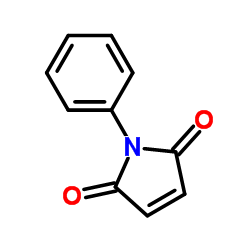 |
N-Phenylmaleimide
CAS:941-69-5 |