A direct-colouring, metal precipitation method for the demonstration of arylsulphatases A and B.
S Partanen
Index: Histochem. J. 16(5) , 501-6, (1984)
Full Text: HTML
Abstract
A new, direct-colouring, metal precipitation method for the light microscopical demonstration of arylsulphatases A and B is described. It is based on the reducing capacity of nitrocatechol liberated by arylsulphatases from p-nitrocatechol sulphate. The reaction is carried out in Karnovsky-Roots' copper ferricyanide incubation mixture at pH 5.0 or 5.5; the nitrocatechol liberated reduces ferricyanide to ferrocyanide, which in turn forms a brown precipitate with copper that indicates enzyme activity. Enzyme activity was localized in cytoplasmic particles compatible with a lysosomal localization of the enzyme. The histochemical reaction was inhibited by phosphate, which has been shown to inhibit arylsulphatases A and B in biochemical determinations. The method described is technically simple, and sections can be mounted in synthetic resin after dehydration.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
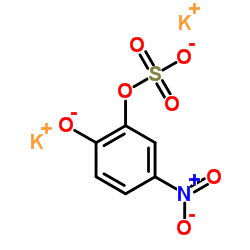 |
Dipotassium 5-nitro-2-oxidophenyl sulfate
CAS:14528-64-4 |
C6H3K2NO7S |
|
Arylsulfatase A bound to poly(butyl cyanoacrylate) nanoparti...
2014-07-01 [Pharmazie 69(7) , 518-24, (2014)] |
|
Investigation of catalytic loop structure, dynamics, and fun...
2012-05-31 [J. Phys. Chem. B 116(21) , 6166-76, (2012)] |
|
Isolation and characterization of rat hepatic ascorbic acid-...
1987-01-01 [Enzyme 37(3) , 134-40, (1987)] |
|
Chemical characterization and substrate specificity of rabbi...
1980-07-10 [Biochim. Biophys. Acta 614(1) , 92-101, (1980)] |
|
Ultrastructural localization of Trypanosoma cruzi lysosomes ...
2007-01-01 [Micron 38(3) , 252-6, (2007)] |