Bioorganicheskaia khimiia
1996-05-01
[Direct transition of S-acetamidomethyl-protected peptide 593-603 of HIV-2 gp41 into a cyclic disulfide using iodine. Study of side reactions].
E V Kudriavtseva, M V Sidorova, M V Ovchinnikov, Zh D Bespalova, V N Bushuev, R P Evstigneeva
Index: Bioorg. Khim. 22(5) , 370-5, (1996)
Full Text: HTML
Abstract
Removal of Acm-protecting group from thiol functional groups of Cys residues with simultaneous disulfide bridge formation by iodine in acetic acid was studied in the course of the synthesis of a peptide fragment corresponding to 593-603 sequence of HIV-2 gp41 glycoprotein. The excess iodine influence on the cyclization process was investigated. By-products of the oxidative disulfide formation were isolated, and their structures were elucidated by means of amino acid and elemental analyses, mass spectrometry, NMR, and UV-spectroscopy.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
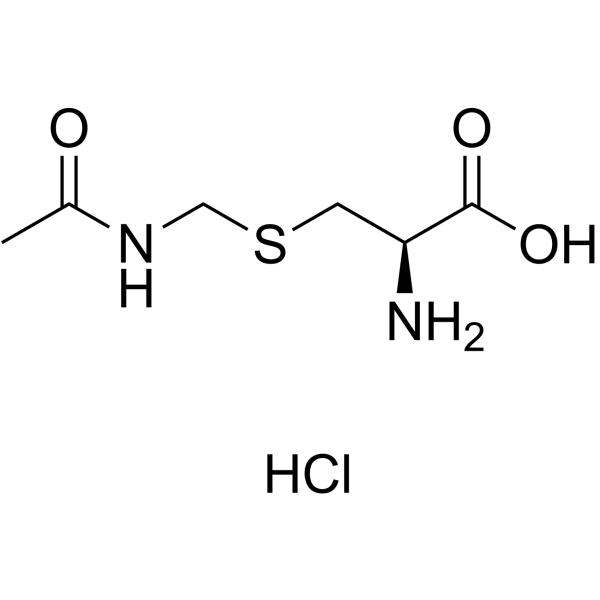 |
H-Cys(Acm)-OH HCl
CAS:28798-28-9 |
C6H13ClN2O3S |
Related Articles:
More...
|
Side reaction during the deprotection of (S-acetamidomethyl)...
1993-01-01 [Int. J. Pept. Protein Res. 41(1) , 85-95, (1993)] |
|
Acetamidomethyl. A novel thiol protecting group for cysteine...
1972-07-26 [J. Am. Chem. Soc. 94 , 5456, (1972)] |
|
Studies on a growth-inhibitory peptide derived from alpha-fe...
2001-01-01 [J. Pept. Res. 57(1) , 29-38, (2001)] |
|
Side reaction of S-to-N acetamidomethyl shift during disulfi...
1995-01-01 [Pept. Res. 8(6) , 316-20, (1995)] |
|
Deprotection of S-acetamidomethyl cysteine-containing peptid...
1995-06-10 [Anal. Biochem. 228(1) , 173-7, (1995)] |