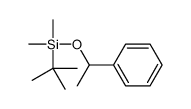
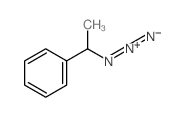
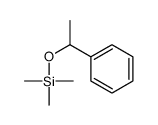
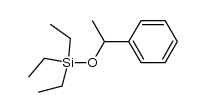
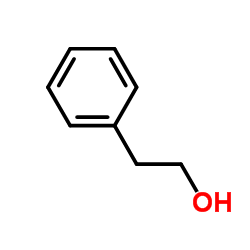
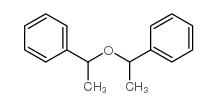
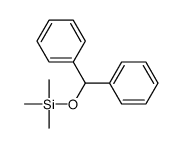
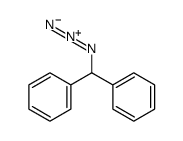
|
~78% |
|
~72% |
|
~73% |
|
~47% |
|
~28% |
|
~21% |
|
~10% |
|
~10% |