Organic Letters
2004-06-24
Total synthesis of natural (+)-(2's,3'r)-zoapatanol.
Catherine Taillier, Véronique Bellosta, Janine Cossy
Index: Org. Lett. 6(13) , 2149-51, (2004)
Full Text: HTML
Abstract
[reaction: see text] (+)-Zoapatanol was synthesized by using four key-steps: a Suzuki cross-coupling to prepare a (Z)-alpha,beta-unsaturated ester followed by an enantioselective dihydroxylation to control the C2' and C3' stereocenters, an intramolecular Horner-Wadsworth-Emmons olefination to construct the oxepane ring, and a chemoselective nucleophilic addition/Birch reduction process of a Weinreb amide to introduce simultaneously the beta,gamma-unsaturated ketone on the side-chain and regenerate alcohols from benzyl ethers.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
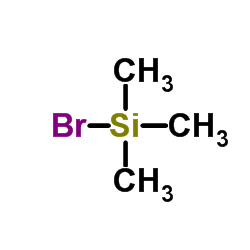 |
Bromo(trimethyl)silane
CAS:2857-97-8 |
C3H9BrSi |
Related Articles:
More...
|
EPR and NMR spectroscopies provide input on the coordination...
2015-06-01 [J. Biol. Inorg. Chem. 20 , 719-27, (2015)] |
|
Position and length of fatty acids strongly affect receptor ...
2014-11-01 [ChemMedChem 9(11) , 2463-74, (2014)] |
|
Reduced vascular endothelial growth factor and capillary den...
2014-07-01 [Brain Pathol. 24(4) , 334-43, (2014)] |
|
Two-step hard acid deprotection/cleavage procedure for solid...
1991-02-01 [Int. J. Pept. Protein Res. 37(2) , 145-52, (1991)] |
|
The chemoselective and efficient deprotection of silyl ether...
2008-06-21 [Org. Biomol. Chem. 6(12) , 2168-72, (2008)] |