Interaction of the brain-specific protein p42IP4/centaurin-alpha1 with the peptidase nardilysin is regulated by the cognate ligands of p42IP4, PtdIns(3,4,5)P3 and Ins(1,3,4,5)P4, with stereospecificity.
Rolf Stricker, K Martin Chow, Daniela Walther, Theodor Hanck, Louis B Hersh, Georg Reiser
Index: J. Neurochem. 98(2) , 343-54, (2006)
Full Text: HTML
Abstract
The brain-specific protein p42IP4, also called centaurin-alpha1, specifically binds phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] and inositol 1,3,4,5-tetrakisphosphate [Ins(1,3,4,5)P4]. Here, we investigate the interaction of p42IP4/centaurin-alpha1 with nardilysin (NRDc), a member of the M16 family of zinc metalloendopeptidases. Members of this peptidase family exhibit enzymatic activity and also act as receptors for other proteins. We found that p42IP4/centaurin-alpha1 binds specifically to NRDc from rat brain. We further detected that centaurin-alpha2, a protein that is highly homologous to p42IP4/centaurin-alpha1 and expressed ubiquitously, also binds to NRDc. In vivo interaction was demonstrated by co-immunoprecipitation of p42IP4/centaurin-alpha1 with NRDc from rat brain. The acidic domain of NRDc (NRDc-AD), which does not participate in catalysis, is sufficient for the protein interaction with p42IP4. Interestingly, preincubation of p42IP4 with its cognate ligands D-Ins(1,3,4,5)P4 and the lipid diC8PtdIns(3,4,5)P3 negatively modulates the interaction between the two proteins. D-Ins(1,3,4,5)P4 and diC8PtdIns(3,4,5)P3 suppress the interaction with virtually identical concentration dependencies. This inhibition is highly ligand specific. The enantiomer L-Ins(1,3,4,5)P4 is not effective. Similarly, the phosphoinositides diC8PtdIns(3,4)P2, diC8PtdIns(3,5)P2 and diC8PtdIns(4,5)P2 all have no influence on the interaction. Further experiments revealed that endogenous p42IP4 from rat brain binds to glutathione-S-transferase (GST)-NRDc-AD. The proteins dissociate from each other when incubated with D-Ins(1,3,4,5)P4, but not with inositol 1,4,5-trisphosphate [Ins(1,4,5)P3]. In summary, we demonstrate that p42IP4 binds to NRDc via the NRDc-AD, and that this interaction is controlled by the cognate cellular ligands of p42IP4/centaurin-alpha1. Thus, specific ligands of p42IP4 can modulate the recruitment of proteins, which are docked to p42IP4, to specific cellular compartments.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
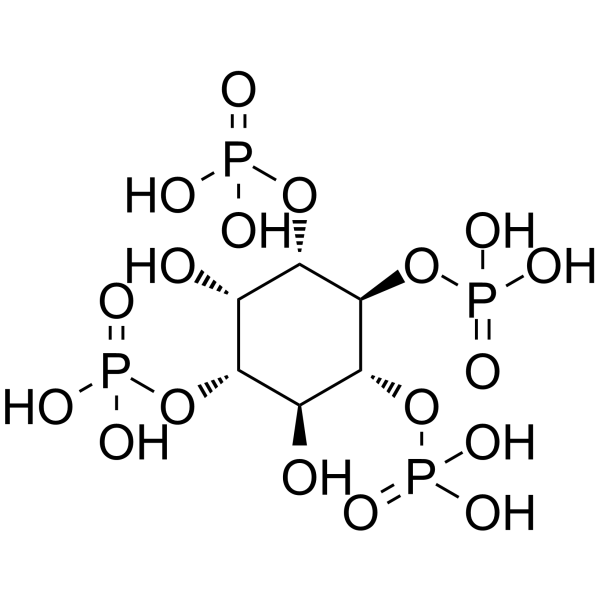 |
Inositol 1,3,4,5-tetraphosphate
CAS:102850-29-3 |
C6H16O18P4 |
|
IP3 3-kinase B controls hematopoietic stem cell homeostasis ...
2015-04-30 [Blood 125 , 2786-97, (2015)] |
|
Cell signaling. The art of the soluble.
2007-05-11 [Science 316(5826) , 845-6, (2007)] |
|
Inositol 1,4,5-trisphosphate 3-kinase B is a negative regula...
2009-04-15 [J. Immunol. 182(8) , 4696-704, (2009)] |
|
Regioselective deprotection of orthobenzoates for the synthe...
2009-04-21 [Org. Biomol. Chem. 7(8) , 1709-15, (2009)] |
|
Beyond IP3: roles for higher order inositol phosphates in im...
2008-02-15 [Cell Cycle 7(4) , 463-7, (2008)] |