Development of diversified methods for chemical modification of the 5,6-double bond of uracil derivatives depending on active methylene compounds.
Hironao Sajiki, Yusuke Iida, Kanoko Ikawa, Yoshinari Sawama, Yasunari Monguchi, Yukio Kitade, Yoshifumi Maki, Hideo Inoue, Kosaku Hirota
Index: Molecules 17(6) , 6519-46, (2012)
Full Text: HTML
Abstract
The reaction of 5-halogenouracil and uridine derivatives 1 and 7 with active methylene compounds under basic conditions produced diverse and selective C-C bond formation products by virtue of the nature of the carbanions. Three different types of reactions such as the regioselective C-C bond formation at the 5- and 6-positions of uracil and uridine derivatives (products 2, 5, 8, 17, 20 and 21), and the formation of fused heterocycle derivatives 2,4-diazabicyclo[4.1.0]heptane (15) and 2,4-diazabicyclo-[4.1.0]nonane (16) via dual C-C bond formations at both the 5- and 6-positions were due to the different active methylene compounds used as reagents.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
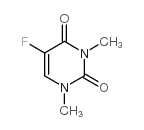 |
5-fluoro-1,3-dimethyluracil
CAS:3013-92-1 |
C6H7FN2O2 |
|
Studies on oxidative modifications of nucleic acid pyrimidin...
1984-01-01 [Nucleic Acids Symp. Ser. (15) , 1-4, (1984)] |
|
Stereoselective synthesis of tetrahydronaphthocyclobuta[1,2-...
2005-02-01 [Chem. Pharm. Bull. 53(2) , 258-9, (2005)] |
|
Structures of three photodimers of 5-fluoro-1,3-dimethylurac...
[Acta Crystallogr. C 40(11) , 1957-60, (1984)] |
|
Enthalpy of formation of 5-fluoro-1,3-dimethyluracil: 5-Fluo...
[J. Chem. Thermodyn. 75 , 106-115, (2014)] |
|
Acid-Catalyzed Photosubstitution of 5-Fluoro-1,3-Dimethylura...
[Nucleosides Nucleotides 11(2-4) , 521-527, (1992)] |