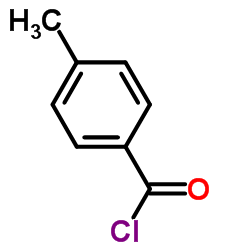
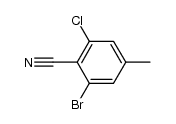
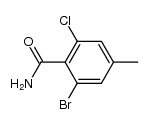
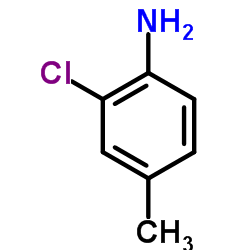
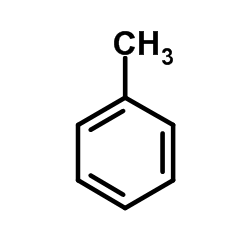
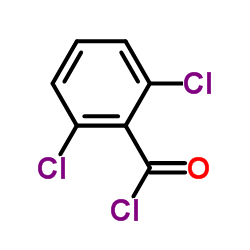
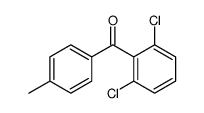
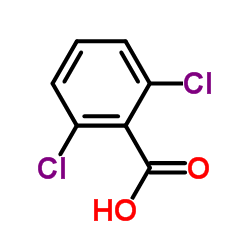
|
~% |
|
~% |
|
~64% |
|
~% |
|
~% |
|
~% |
|
~64% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
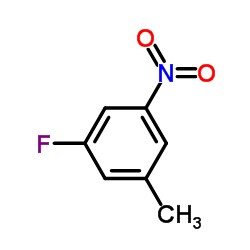
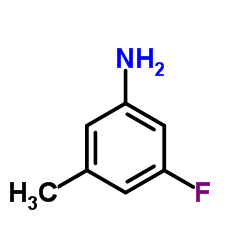

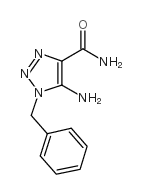
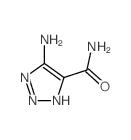
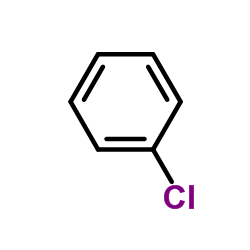
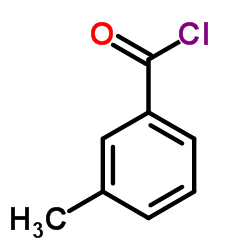
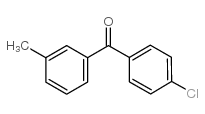


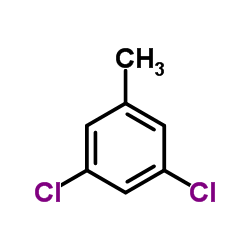
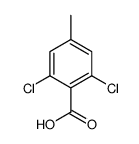
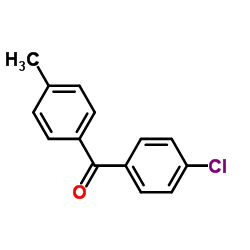
![[4-(bromomethyl)phenyl]-(4-chlorophenyl)methanone Structure](https://image.chemsrc.com/caspic/421/91457-11-3.png)