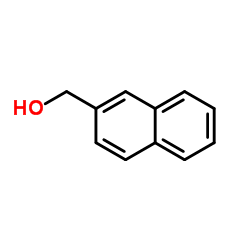
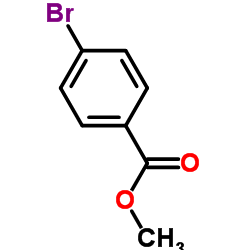
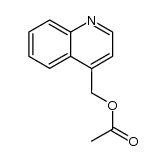
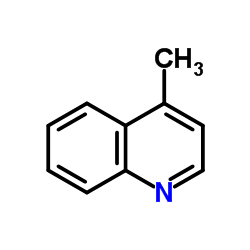
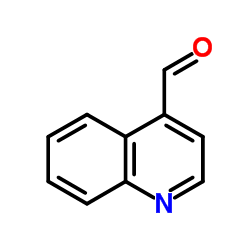
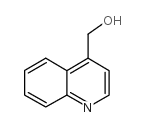
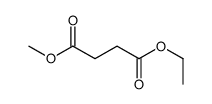
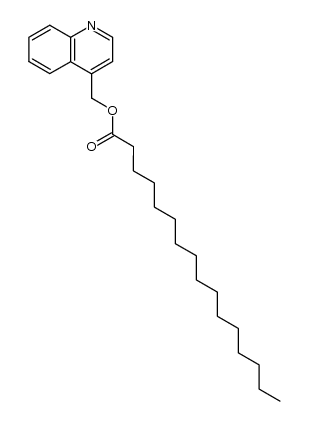
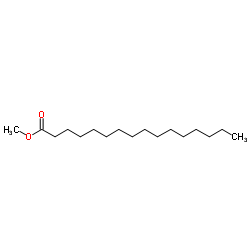
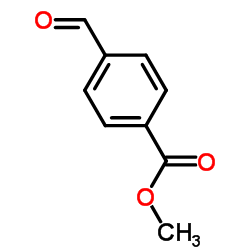
|
~% |
|
~95% |
|
~87% |
|
~% |
|
~10% |
|
~88% |
|
~93% |
|
~92% |
|
~93% |