An efficient synthesis of an alphavbeta3 antagonist.
Nobuyoshi Yasuda, Yi Hsiao, Mark S Jensen, Nelo R Rivera, Chunhua Yang, Kenneth M Wells, James Yau, Michael Palucki, Lushi Tan, Peter G Dormer, Ralph P Volante, David L Hughes, Paul J Reider
Index: J. Org. Chem. 69 , 1959-1966, (2004)
Full Text: HTML
Abstract
A practical preparation of an alpha(v)beta(3) antagonist is reported. The antagonist consists of three key components, a tetrahydronaphthyridine moiety, a beta-alanine moiety, and a central imidazolidone moiety. The tetrahydronaphthyridine component was prepared using two different methods, both of which relied on variations of the Friedländer reaction to establish the desired regiochemistry. The beta-alanine component was prepared using Davies' asymmetric 1,4-addition methodology as the key stereo-defining step. The central imidazolidone portion was created from these two components using an effective three-step cyclization protocol. Thus, a highly convergent process for the drug candidate was defined.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
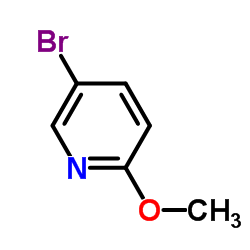 |
2-Methoxy-5-Bromopyridine
CAS:13472-85-0 |
C6H6BrNO |
|
Non-peptide alpha v beta 3 antagonists. Part 7: 3-Substitute...
2004-02-23 [Bioorg. Med. Chem. Lett. 14 , 1049, (2004)] |
|
[Tetrahedron Asymmetry 14 , 3469, (2003)] |
|
The 1,3-Diaminobenzene-Derived Aminophosphine Palladium Pinc...
[Adv. Synth. Catal. 352(6) , 1075-1080, (2010)] |
|
Functionalized pyridylboronic acids and their Suzuki cross-c...
[J. Org. Chem. 67(21) , 7541-7543, (2002)] |
|
A scaleable synthesis of methyl 3-amino-5-(4-fluorobenzyl)-2...
[Org. Process Res. Dev. 11(5) , 899-902, (2007)] |