Lysine-bisglycidol conjugates as novel lysine cationic surfactants.
Aurora Pinazo, Marta Angelet, Ramon Pons, Marina Lozano, Ma Rosa Infante, Lourdes Pérez
Index: Langmuir 25 , 7803-7814, (2009)
Full Text: HTML
Abstract
The synthesis of a novel class of lysine-based cationic amphiphilic derivatives of the type N(epsilon),N(epsilon)'-bis(n-acyloxypropyl)-l-lysine methyl ester salts combining several hydroxyl functions and aliphatic chains of 12 or 14 carbon atoms is described. The compounds have one, two, three, and four alkyl chains. Aggregation in water was studied by four different methods: surface tension, conductivity, chloride ion activity, and nuclear magnetic resonance ((1)H NMR). The critical aggregation concentration value of the new surfactants depends on both the number of alkyl chains and the alkyl chain length. The formation of vesicles at low concentrations was confirmed by H(1) NMR and optical microscopy. Antimicrobial activity was determined on the basis of the minimum inhibitory concentration (MIC) values. These novel lysine-based surfactants showed moderate antimicrobial activity which may be an important advantage for many biomedical applications.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
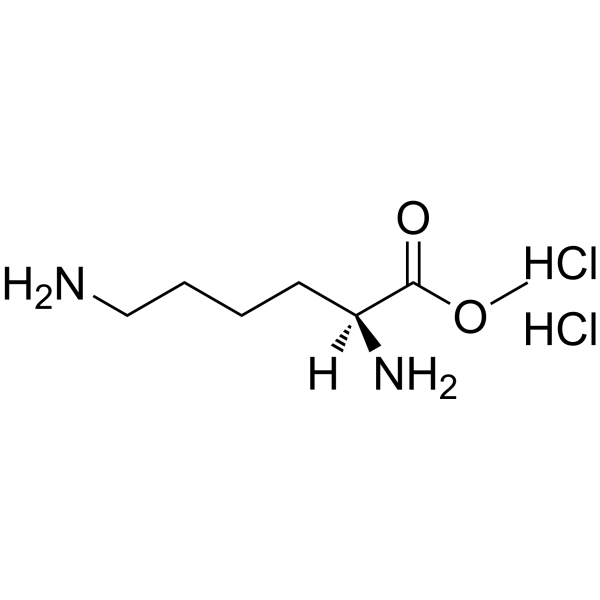 |
H-Lys-OMe.2HCl
CAS:26348-70-9 |
C7H18Cl2N2O2 |
|
pH-responsive, lysine-based hydrogels for the oral delivery ...
2015-01-30 [Int. J. Pharm. 478(2) , 496-503, (2015)] |
|
A neutral polydisulfide containing Gd(III) DOTA monoamide as...
2016-01-01 [Contrast Media Mol. Imaging 11 , 32-40, (2016)] |
|
Characterization of the chemical degradation of hyaluronic a...
2007-12-28 [Carbohydr. Res. 342 , 2776-2792, (2007)] |
|
Synthesis, physical and biological properties of lithocholyl...
1997-10-20 [Biochim. Biophys. Acta 1336 , 485-496, (1997)] |