Dexamethasone-isonicotinate aerosol in the long-term treatment of steroid-dependent asthmatic children.
M A Manicatide, V V Nicolaescu, V Stroescu, M Voiculescu
Index: Med. Interne 18(2) , 203-10, (1980)
Full Text: HTML
Abstract
Dexamethasone-isonicotinate aerosol (DIA) was administered to twenty-nine steroid-dependent children with chronic perennial asthma, in an open trial during one year. An attempt was made to withdraw systemic corticosteroid therapy. As judged by clinical results, inhaled dexamethasone-isonicotinate controlled the asthma quite as well as did previous therapy. Oral corticosteroids were withdrawn in 27 patients, and the dosage considerably reduced in another two. Cushingoid features subsided. There was a statistically significant improvement in most pulmonary function parameters (airway obstruction and lung hyperinflation were reduced towards normal). Reduction of systemic steroid dosage resulted in the appearance of previously suppressed manifestations such as hay fever, eczema, and nasal polyps. There was no increased tendency to the occurrence of respiratory tract infection; routine examination showed Candida albicans in about 37% cultures.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
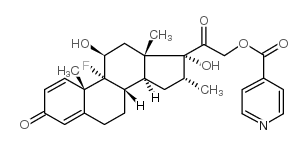 |
dexamethasone isonicotinate
CAS:2265-64-7 |
C28H32FNO6 |
|
Glucose concentrations in plasma and urine after glucocortic...
1982-01-01 [Nord. Vet. Med. 34(4-5) , 143-6, (1982)] |
|
Dexamethasone isonicotinate inhibits dual and late asthmatic...
1989-10-01 [Ann. Allergy 63(4) , 292-6, (1989)] |
|
Effect of dexamethasone isonicotinate on milk yield in ketot...
1991-05-04 [Vet. Rec. 128(18) , 427, (1991)] |
|
A comparison of botulinum toxin a and intralesional steroids...
2013-01-01 [Foot Ankle Int. 34(1) , 8-14, (2013)] |
|
Action of dexamethasone in an equine model of acute non-immu...
1990-01-01 [Res. Vet. Sci. 48(1) , 87-95, (1990)] |