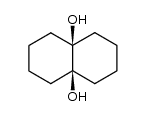
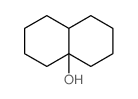
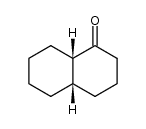
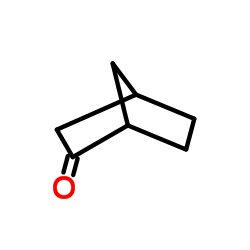
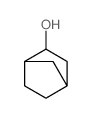
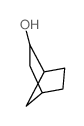
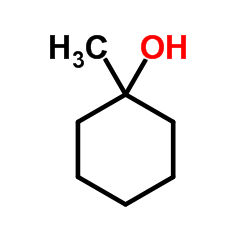
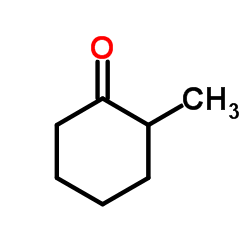
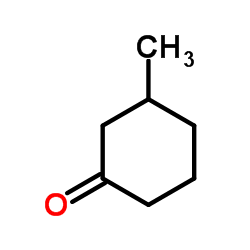
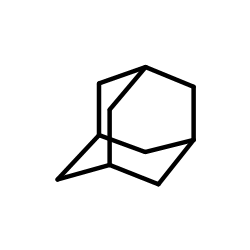
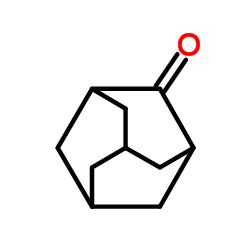
|
~90%
Detail
|
|
~% |
|
~% |
|
~0% |
|
~% |
|
~0% |
|
~0% |
|
~86% |
|
~6% |
|
~% |
|
~97% |